Finerenone cardiovascular and kidney outcomes by age and sex: FIDELITY post hoc analysis of two phase 3, multicentre, double-blind trials
- PMID: 38508632
- PMCID: PMC10952937
- DOI: 10.1136/bmjopen-2023-076444
Finerenone cardiovascular and kidney outcomes by age and sex: FIDELITY post hoc analysis of two phase 3, multicentre, double-blind trials
Abstract
Objectives: This study aimed to evaluate the efficacy and safety of finerenone, a selective, non-steroidal mineralocorticoid receptor antagonist, on cardiovascular and kidney outcomes by age and/or sex.
Design: FIDELITY post hoc analysis; median follow-up of 3 years.
Setting: FIDELITY: a prespecified analysis of the FIDELIO-DKD and FIGARO-DKD trials.
Participants: Adults with type 2 diabetes and chronic kidney disease receiving optimised renin-angiotensin system inhibitors (N=13 026).
Interventions: Randomised 1:1; finerenone or placebo.
Primary and secondary outcome measures: Cardiovascular (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke or hospitalisation for heart failure (HHF)) and kidney (kidney failure, sustained ≥57% estimated glomerular filtration rate (eGFR) decline or renal death) composite outcomes.
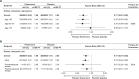
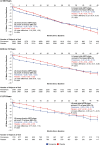
Results: Mean age was 64.8 years; 45.2%, 40.1% and 14.7% were aged <65, 65-74 and ≥75 years, respectively; 69.8% were male. Cardiovascular benefits of finerenone versus placebo were consistent across age (HR 0.94 (95% CI 0.81 to 1.10) (<65 years), HR 0.84 (95% CI 0.73 to 0.98) (65-74 years), HR 0.80 (95% CI 0.65 to 0.99) (≥75 years); Pinteraction=0.42) and sex categories (HR 0.86 (95% CI 0.77 to 0.96) (male), HR 0.89 (95% CI 0.35 to 2.27) (premenopausal female), HR 0.87 (95% CI 0.73 to 1.05) (postmenopausal female); Pinteraction=0.99). Effects on HHF reduction were not modified by age (Pinteraction=0.70) but appeared more pronounced in males (Pinteraction=0.02). Kidney events were reduced with finerenone versus placebo in age groups <65 and 65-74 but not ≥75; no heterogeneity in treatment effect was observed (Pinteraction=0.51). In sex subgroups, finerenone consistently reduced kidney events (Pinteraction=0.85). Finerenone reduced albuminuria and eGFR decline regardless of age and sex. Hyperkalaemia increased with finerenone, but discontinuation rates were <3% across subgroups. Gynaecomastia in males was uncommon across age subgroups and identical between treatment groups.
Conclusions: Finerenone improved cardiovascular and kidney composite outcomes with no significant heterogeneity between age and sex subgroups; however, the effect on HHF appeared more pronounced in males. Finerenone demonstrated a similar safety profile across age and sex subgroups.
Trial registration numbers: NCT02540993, NCT02545049.
Keywords: cardiovascular disease; diabetes & endocrinology; diabetic nephropathy & vascular disease; risk factors.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SB reports research support from 3ive, Bayer, Boehringer Ingelheim, Novartis and Novo Nordisk; honorarium from UpToDate; consultancy fees from Baxter; and speaker bureau fees from Home Dialysis University and PD Excellence Academy. MEFC reports consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, and Fresenius Medical Care, and research support from Baxter and Fresenius. RB reports consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, MSD, Mundipharma, Sanofi and Servier. SDA reports grants from Abbott Vascular and Vifor International; and personal fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BioVentrix, Brahms, Cardiac Dimensions, Cardior, Cordio, CVRx, Edwards, Impulse Dynamics, Janssen, Novartis, Occlutech, Respicardia, Servier, Vectorious and V-Wave. GLB reports consultancy fees from Alnylam, Ionis and Merck. GF is a trial committee member for Amgen, Bayer (no fees received), Boehringer Ingelheim, Medtronic, Novartis, Servier and Vifor. PR reports personal fees from Bayer during the conduct of the study; he has received research support and personal fees from AstraZeneca and Novo Nordisk, and personal fees from Astellas, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, Sanofi and Vifor; all fees are given to Steno Diabetes Center Copenhagen. LMR reports consultancy fees from Bayer. AEF, PK, AL and MB are all full-time employees of Bayer. BP reports consultant fees for AstraZeneca, Bayer, Boehringer Ingelheim, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, PhaseBio, Proton Intel, Sanofi/Lexicon, Sarfez, scPharmaceuticals, SQ Innovation, Tricida, and Vifor/Relypsa; he has stock options for Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, Proton Intel, Sarfez, scPharmaceuticals, SQ Innovation, Tricida, and Vifor/Relypsa; he also holds a patent for site-specific delivery of eplerenone to the myocardium (US patent #9931412) and a provisional patent for histone-acetylation-modulating agents for the treatment and prevention of organ injury (provisional patent US 63/045784).
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
