Strong positive selection biases identity-by-descent-based inferences of recent demography and population structure in Plasmodium falciparum
- PMID: 38509066
- PMCID: PMC10954658
- DOI: 10.1038/s41467-024-46659-0
Strong positive selection biases identity-by-descent-based inferences of recent demography and population structure in Plasmodium falciparum
Abstract
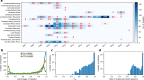
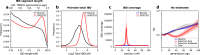
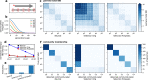
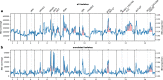
Malaria genomic surveillance often estimates parasite genetic relatedness using metrics such as Identity-By-Decent (IBD), yet strong positive selection stemming from antimalarial drug resistance or other interventions may bias IBD-based estimates. In this study, we use simulations, a true IBD inference algorithm, and empirical data sets from different malaria transmission settings to investigate the extent of this bias and explore potential correction strategies. We analyze whole genome sequence data generated from 640 new and 3089 publicly available Plasmodium falciparum clinical isolates. We demonstrate that positive selection distorts IBD distributions, leading to underestimated effective population size and blurred population structure. Additionally, we discover that the removal of IBD peak regions partially restores the accuracy of IBD-based inferences, with this effect contingent on the population's background genetic relatedness and extent of inbreeding. Consequently, we advocate for selection correction for parasite populations undergoing strong, recent positive selection, particularly in high malaria transmission settings.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Update of
-
Strong Positive Selection Biases Identity-By-Descent-Based Inferences of Recent Demography and Population Structure in Plasmodium falciparum.bioRxiv [Preprint]. 2023 Jul 15:2023.07.14.549114. doi: 10.1101/2023.07.14.549114. bioRxiv. 2023. Update in: Nat Commun. 2024 Mar 20;15(1):2499. doi: 10.1038/s41467-024-46659-0. PMID: 37502843 Free PMC article. Updated. Preprint.
References
-
- World Health Organization. World Malaria Report 2022 (World Health Organization, 2022).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

