Structure of antiviral drug bulevirtide bound to hepatitis B and D virus receptor protein NTCP
- PMID: 38509088
- PMCID: PMC10954734
- DOI: 10.1038/s41467-024-46706-w
Structure of antiviral drug bulevirtide bound to hepatitis B and D virus receptor protein NTCP
Abstract
Cellular entry of the hepatitis B and D viruses (HBV/HDV) requires binding of the viral surface polypeptide preS1 to the hepatobiliary transporter Na+-taurocholate co-transporting polypeptide (NTCP). This interaction can be blocked by bulevirtide (BLV, formerly Myrcludex B), a preS1 derivative and approved drug for treating HDV infection. Here, to elucidate the basis of this inhibitory function, we determined a cryo-EM structure of BLV-bound human NTCP. BLV forms two domains, a plug lodged in the bile salt transport tunnel of NTCP and a string that covers the receptor's extracellular surface. The N-terminally attached myristoyl group of BLV interacts with the lipid-exposed surface of NTCP. Our structure reveals how BLV inhibits bile salt transport, rationalizes NTCP mutations that decrease the risk of HBV/HDV infection, and provides a basis for understanding the host specificity of HBV/HDV. Our results provide opportunities for structure-guided development of inhibitors that target HBV/HDV docking to NTCP.
© 2024. The Author(s).
Conflict of interest statement
Stephan Urban is the inventor of and holds patents on Bulevirtide, under the patent number WO2019219840A1. The remaining authors declare no competing interests.
Figures




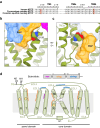
References
MeSH terms
Substances
Grants and funding
- R01 GM117372/GM/NIGMS NIH HHS/United States
- 214834/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 197785619/Deutsche Forschungsgemeinschaft (German Research Foundation)
- GM117372/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Medical

