Oxytoxaceae are prorocentralean rather than peridinialean dinophytes and taxonomic clarification of heterotrophic Oxytoxum lohmannii (≡ "Amphidinium" crassum) by epitypification
- PMID: 38509105
- PMCID: PMC10954643
- DOI: 10.1038/s41598-024-56848-y
Oxytoxaceae are prorocentralean rather than peridinialean dinophytes and taxonomic clarification of heterotrophic Oxytoxum lohmannii (≡ "Amphidinium" crassum) by epitypification
Abstract
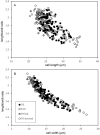
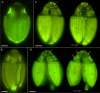
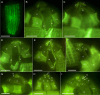
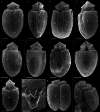
During evolution of Dinophyceae, size reduction of the episome has occurred in several lineages (including unarmoured Amphidiniales and armoured Prorocentrales). One such species is Amphidinium crassum, whose taxonomic identity is elusive though showing morphological similarities with Oxytoxaceae (currently placed in armoured Peridiniales). Plankton samples were taken at the type locality of A. crassum in Kiel Bight (Baltic Sea) in order to establish monoclonal strains. The protist material was examined in detail using light and electron microscopy, and a long (2984 bp) ribosomal RNA sequence gained was part of a taxon sample comprising 206 specimen vouchers and representing the known molecular diversity of Dinophyceae. Cells of A. crassum were ovoid and exhibited a plate pattern po, 4', 1a, 6'', 5c, 4s, 5''', 1''''. In the molecular phylogeny, the species seemed to belong neither to Amphidiniales nor to Peridiniales but to Prorocentrales and clustered with other representatives of Oxytoxaceae. The morphological diversity of Prorocentrales appears thus expanded, and the group may include a number of previously unrecognised representatives unusually having five postcingular and only a single antapical plate. The taxonomic identity of A. crassum is clarified by epitypification, and the species notably exhibits both an apical pore and an additional epithecal pore.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

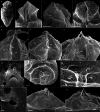
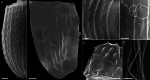
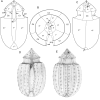

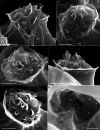
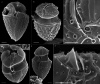


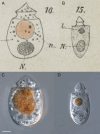
Similar articles
-
Plate pattern clarification of the marine dinophyte Heterocapsa triquetra sensu Stein (Dinophyceae) collected at the Kiel Fjord (Germany).J Phycol. 2017 Dec;53(6):1305-1324. doi: 10.1111/jpy.12584. Epub 2017 Oct 10. J Phycol. 2017. PMID: 28915316
-
Spatial fragmentation in the distribution of diatom endosymbionts from the taxonomically clarified dinophyte Kryptoperidinium triquetrum (= Kryptoperidinium foliaceum, Peridiniales).Sci Rep. 2023 May 26;13(1):8593. doi: 10.1038/s41598-023-32949-y. Sci Rep. 2023. PMID: 37237053 Free PMC article.
-
The Hot Spot in a Cold Environment: Puzzling Parvodinium (Peridiniopsidaceae, Peridiniales) from the Polish Tatra Mountains.Protist. 2018 Apr;169(2):206-230. doi: 10.1016/j.protis.2018.02.004. Epub 2018 Feb 23. Protist. 2018. PMID: 29631116
-
Fensomea setacea, gen. & sp. nov. (Cladopyxidaceae, Dinophyceae), is neither gonyaulacoid nor peridinioid as inferred from morphological and molecular data.Sci Rep. 2021 Jun 17;11(1):12824. doi: 10.1038/s41598-021-92107-0. Sci Rep. 2021. PMID: 34140573 Free PMC article.
-
The molecular ecology of microbial eukaryotes unveils a hidden world.Trends Microbiol. 2002 Jan;10(1):31-8. doi: 10.1016/s0966-842x(01)02257-0. Trends Microbiol. 2002. PMID: 11755083 Review.
Cited by
-
An Update on the Morphology and Phylogeny of the Nanoplanktonic Dinoflagellate Prorocentrum nux.J Eukaryot Microbiol. 2025 Jul-Aug;72(4):e70019. doi: 10.1111/jeu.70019. J Eukaryot Microbiol. 2025. PMID: 40566962 Free PMC article.
References
-
- Gómez F. A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata) CICIMAR Oceánides. 2012;27:65–140. doi: 10.37543/oceanides.v27i1.111. - DOI
-
- Takayama H. Apical grooves of unarmored dinoflagellates. Bull. Plankton Soc. Jpn. 1985;32:129–140.
-
- Daugbjerg N, Hansen G, Larsen J, Moestrup Ø. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia. 2000;39:302–317. doi: 10.2216/i0031-8884-39-4-302.1. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

