Sulfide oxidation promotes hypoxic angiogenesis and neovascularization
- PMID: 38509349
- PMCID: PMC11584973
- DOI: 10.1038/s41589-024-01583-8
Sulfide oxidation promotes hypoxic angiogenesis and neovascularization
Abstract
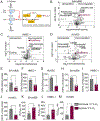
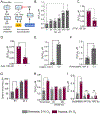
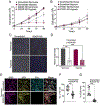
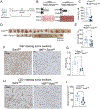
Angiogenic programming in the vascular endothelium is a tightly regulated process for maintaining tissue homeostasis and is activated in tissue injury and the tumor microenvironment. The metabolic basis of how gas signaling molecules regulate angiogenesis is elusive. Here, we report that hypoxic upregulation of ·NO in endothelial cells reprograms the transsulfuration pathway to increase biogenesis of hydrogen sulfide (H2S), a proangiogenic metabolite. However, decreased H2S oxidation due to sulfide quinone oxidoreductase (SQOR) deficiency synergizes with hypoxia, inducing a reductive shift and limiting endothelial proliferation that is attenuated by dissipation of the mitochondrial NADH pool. Tumor xenografts in whole-body (WBCreSqorfl/fl) and endothelial-specific (VE-cadherinCre-ERT2Sqorfl/fl) Sqor-knockout mice exhibit lower mass and angiogenesis than control mice. WBCreSqorfl/fl mice also exhibit decreased muscle angiogenesis following femoral artery ligation compared to control mice. Collectively, our data reveal the molecular intersections between H2S, O2 and ·NO metabolism and identify SQOR inhibition as a metabolic vulnerability for endothelial cell proliferation and neovascularization.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures

Update of
-
Sulfide oxidation promotes hypoxic angiogenesis and neovascularization.bioRxiv [Preprint]. 2023 Mar 15:2023.03.14.532677. doi: 10.1101/2023.03.14.532677. bioRxiv. 2023. Update in: Nat Chem Biol. 2024 Oct;20(10):1294-1304. doi: 10.1038/s41589-024-01583-8. PMID: 36993187 Free PMC article. Updated. Preprint.
References
-
- Carmeliet P. Angiogenesis in health and disease. Nat Med 9, 653–60 (2003). - PubMed
-
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature 438, 932–6 (2005). - PubMed
-
- Folkman J. Angiogenesis. Annu Rev Med 57, 1–18 (2006). - PubMed
-
- Hurwitz H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350, 2335–42 (2004). - PubMed
MeSH terms
Substances
Grants and funding
- R01 CA148828/CA/NCI NIH HHS/United States
- R01 DK095201/DK/NIDDK NIH HHS/United States
- R35GM130183/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 CA248160/CA/NCI NIH HHS/United States
- T32 GM007863/GM/NIGMS NIH HHS/United States
- R01CA245546/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- T32 GM132046/GM/NIGMS NIH HHS/United States
- R01CA248160/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01CA148828/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 CA245546/CA/NCI NIH HHS/United States
- R35 GM130183/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

