Compensatory evolution in NusG improves fitness of drug-resistant M. tuberculosis
- PMID: 38509362
- PMCID: PMC10990936
- DOI: 10.1038/s41586-024-07206-5
Compensatory evolution in NusG improves fitness of drug-resistant M. tuberculosis
Abstract
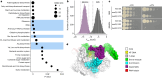
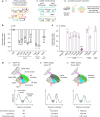
Drug-resistant bacteria are emerging as a global threat, despite frequently being less fit than their drug-susceptible ancestors1-8. Here we sought to define the mechanisms that drive or buffer the fitness cost of rifampicin resistance (RifR) in the bacterial pathogen Mycobacterium tuberculosis (Mtb). Rifampicin inhibits RNA polymerase (RNAP) and is a cornerstone of modern short-course tuberculosis therapy9,10. However, RifR Mtb accounts for one-quarter of all deaths due to drug-resistant bacteria11,12. We took a comparative functional genomics approach to define processes that are differentially vulnerable to CRISPR interference (CRISPRi) inhibition in RifR Mtb. Among other hits, we found that the universally conserved transcription factor NusG is crucial for the fitness of RifR Mtb. In contrast to its role in Escherichia coli, Mtb NusG has an essential RNAP pro-pausing function mediated by distinct contacts with RNAP and the DNA13. We find this pro-pausing NusG-RNAP interface to be under positive selection in clinical RifR Mtb isolates. Mutations in the NusG-RNAP interface reduce pro-pausing activity and increase fitness of RifR Mtb. Collectively, these results define excessive RNAP pausing as a molecular mechanism that drives the fitness cost of RifR in Mtb, identify a new mechanism of compensation to overcome this cost, suggest rational approaches to exacerbate the fitness cost, and, more broadly, could inform new therapeutic approaches to develop drug combinations to slow the evolution of RifR in Mtb.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

