Venous-plexus-associated lymphoid hubs support meningeal humoral immunity
- PMID: 38509366
- PMCID: PMC11482273
- DOI: 10.1038/s41586-024-07202-9
Venous-plexus-associated lymphoid hubs support meningeal humoral immunity
Abstract
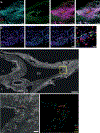
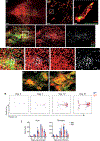
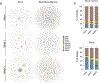
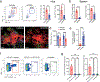
There is increasing interest in how immune cells in the meninges-the membranes that surround the brain and spinal cord-contribute to homeostasis and disease in the central nervous system1,2. The outer layer of the meninges, the dura mater, has recently been described to contain both innate and adaptive immune cells, and functions as a site for B cell development3-6. Here we identify organized lymphoid structures that protect fenestrated vasculature in the dura mater. The most elaborate of these dural-associated lymphoid tissues (DALT) surrounded the rostral-rhinal confluence of the sinuses and included lymphatic vessels. We termed this structure, which interfaces with the skull bone marrow and a comparable venous plexus at the skull base, the rostral-rhinal venolymphatic hub. Immune aggregates were present in DALT during homeostasis and expanded with age or after challenge with systemic or nasal antigens. DALT contain germinal centre B cells and support the generation of somatically mutated, antibody-producing cells in response to a nasal pathogen challenge. Inhibition of lymphocyte entry into the rostral-rhinal hub at the time of nasal viral challenge abrogated the generation of germinal centre B cells and class-switched plasma cells, as did perturbation of B-T cell interactions. These data demonstrate a lymphoid structure around vasculature in the dura mater that can sample antigens and rapidly support humoral immune responses after local pathogen challenge.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Figures









References
-
- Korin B et al. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat. Neurosci 20, 1300–1309 (2017). - PubMed
-
- Schafflick D et al. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat. Neurosci 24, 1225–1234 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

