Structure and assembly of a bacterial gasdermin pore
- PMID: 38509367
- PMCID: PMC11771145
- DOI: 10.1038/s41586-024-07216-3
Structure and assembly of a bacterial gasdermin pore
Abstract
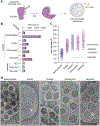
In response to pathogen infection, gasdermin (GSDM) proteins form membrane pores that induce a host cell death process called pyroptosis1-3. Studies of human and mouse GSDM pores have revealed the functions and architectures of assemblies comprising 24 to 33 protomers4-9, but the mechanism and evolutionary origin of membrane targeting and GSDM pore formation remain unknown. Here we determine a structure of a bacterial GSDM (bGSDM) pore and define a conserved mechanism of pore assembly. Engineering a panel of bGSDMs for site-specific proteolytic activation, we demonstrate that diverse bGSDMs form distinct pore sizes that range from smaller mammalian-like assemblies to exceptionally large pores containing more than 50 protomers. We determine a cryo-electron microscopy structure of a Vitiosangium bGSDM in an active 'slinky'-like oligomeric conformation and analyse bGSDM pores in a native lipid environment to create an atomic-level model of a full 52-mer bGSDM pore. Combining our structural analysis with molecular dynamics simulations and cellular assays, our results support a stepwise model of GSDM pore assembly and suggest that a covalently bound palmitoyl can leave a hydrophobic sheath and insert into the membrane before formation of the membrane-spanning β-strand regions. These results reveal the diversity of GSDM pores found in nature and explain the function of an ancient post-translational modification in enabling programmed host cell death.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures




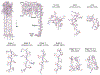







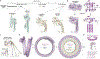

Update of
-
Structure and assembly of a bacterial gasdermin pore.bioRxiv [Preprint]. 2023 Oct 26:2023.04.20.537723. doi: 10.1101/2023.04.20.537723. bioRxiv. 2023. Update in: Nature. 2024 Apr;628(8008):657-663. doi: 10.1038/s41586-024-07216-3. PMID: 37131678 Free PMC article. Updated. Preprint.
References
-
- Broz P, Pelegrín P & Shao F The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol 20, 143–157 (2020). - PubMed
-
- Shi J, Gao W & Shao F Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci 42, 245–254 (2017). - PubMed
-
- Ding J et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

