Iron depletion has different consequences on the growth and survival of Toxoplasma gondii strains
- PMID: 38509723
- PMCID: PMC10962585
- DOI: 10.1080/21505594.2024.2329566
Iron depletion has different consequences on the growth and survival of Toxoplasma gondii strains
Abstract
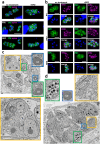
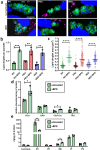
Toxoplasma gondii is an obligate intracellular parasite responsible for a pathology called toxoplasmosis, which primarily affects immunocompromised individuals and developing foetuses. The parasite can scavenge essential nutrients from its host to support its growth and survival. Among them, iron is one of the most important elements needed to sustain basic cellular functions as it is involved in a number of key metabolic processes, including oxygen transport, redox balance, and electron transport. We evaluated the effects of an iron chelator on the development of several parasite strains and found that they differed in their ability to tolerate iron depletion. The growth of parasites usually associated with a model of acute toxoplasmosis was strongly affected by iron depletion, whereas cystogenic strains were less sensitive as they were able to convert into persisting developmental forms that are associated with the chronic form of the disease. Ultrastructural and biochemical characterization of the impact of iron depletion on parasites also highlighted striking changes in both their metabolism and that of the host, with a marked accumulation of lipid droplets and perturbation of lipid homoeostasis. Overall, our study demonstrates that although acute iron depletion has an important effect on the growth of T. gondii, it has a more profound impact on actively dividing parasites, whereas less metabolically active parasite forms may be able to avoid some of the most detrimental consequences.
Keywords: Acute toxoplasmosis; bradyzoites; chronic toxoplasmosis; cystogenic strains; iron depletion.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
The acyl-CoA synthetase TgACS1 allows neutral lipid metabolism and extracellular motility in Toxoplasma gondii through relocation via its peroxisomal targeting sequence (PTS) under low nutrient conditions.mBio. 2024 Apr 10;15(4):e0042724. doi: 10.1128/mbio.00427-24. Epub 2024 Mar 19. mBio. 2024. PMID: 38501871 Free PMC article.
-
The pathogenicity and virulence of Toxoplasma gondii.Virulence. 2021 Dec;12(1):3095-3114. doi: 10.1080/21505594.2021.2012346. Virulence. 2021. PMID: 34895084 Free PMC article.
-
Iron Stress Affects the Growth and Differentiation of Toxoplasma gondii.Int J Mol Sci. 2024 Feb 21;25(5):2493. doi: 10.3390/ijms25052493. Int J Mol Sci. 2024. PMID: 38473741 Free PMC article.
-
IFNs in host defence and parasite immune evasion during Toxoplasma gondii infections.Front Immunol. 2024 Feb 7;15:1356216. doi: 10.3389/fimmu.2024.1356216. eCollection 2024. Front Immunol. 2024. PMID: 38384452 Free PMC article. Review.
-
Invasion and egress by the obligate intracellular parasite Toxoplasma gondii: potential targets for the development of new antiparasitic drugs.Curr Pharm Des. 2007;13(6):641-51. doi: 10.2174/138161207780162854. Curr Pharm Des. 2007. PMID: 17346179 Review.
Cited by
-
The HCF101 protein is an important component of the cytosolic iron-sulfur synthesis pathway in Toxoplasma gondii.PLoS Biol. 2025 Feb 6;23(2):e3003028. doi: 10.1371/journal.pbio.3003028. eCollection 2025 Feb. PLoS Biol. 2025. PMID: 39913537 Free PMC article.
-
Functional dissection of prenyltransferases reveals roles in endocytosis and secretory vacuolar sorting in type 2-ME49 strain of Toxoplasma gondii.Virulence. 2024 Dec;15(1):2432681. doi: 10.1080/21505594.2024.2432681. Epub 2024 Nov 26. Virulence. 2024. PMID: 39569525 Free PMC article.
-
Iron-mediated post-transcriptional regulation in Toxoplasma gondii.PLoS Pathog. 2025 Feb 3;21(2):e1012857. doi: 10.1371/journal.ppat.1012857. eCollection 2025 Feb. PLoS Pathog. 2025. PMID: 39899594 Free PMC article.
-
PTPA Governs Stress-Responsive Differentiation and Metabolic Homeostasis in Toxoplasma gondii.Cells. 2025 Jun 3;14(11):835. doi: 10.3390/cells14110835. Cells. 2025. PMID: 40498010 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
