Electroacupuncture suppresses neuronal ferroptosis to relieve chronic neuropathic pain
- PMID: 38509741
- PMCID: PMC10955159
- DOI: 10.1111/jcmm.18240
Electroacupuncture suppresses neuronal ferroptosis to relieve chronic neuropathic pain
Abstract
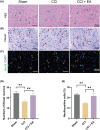
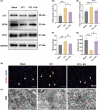
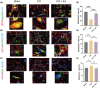
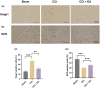
Growing evidence supports the analgesic efficacy of electroacupuncture (EA) in managing chronic neuropathic pain (NP) in both patients and NP models induced by peripheral nerve injury. However, the underlying mechanisms remain incompletely understood. Ferroptosis, a novel form of programmed cell death, has been found to be activated during NP development, while EA has shown potential in promoting neurological recovery following acute cerebral injury by targeting ferroptosis. In this study, to investigate the detailed mechanism underlying EA intervention on NP, male Sprague-Dawley rats with chronic constriction injury (CCI)-induced NP model received EA treatment at acupoints ST36 and GV20 for 14 days. Results demonstrated that EA effectively attenuated CCI-induced pain hypersensitivity and mitigated neuron damage and loss in the spinal cord of NP rats. Moreover, EA reversed the oxidative stress-mediated spinal ferroptosis phenotype by upregulating reduced expression of xCT, glutathione peroxidase 4 (GPX4), ferritin heavy chain (FTH1) and superoxide dismutase (SOD) levels, and downregulating increased expression of acyl-CoA synthetase long-chain family member 4 (ACSL4), malondialdehyde levels and iron overload. Furthermore, EA increased the immunofluorescence co-staining of GPX4 in neurons cells of the spinal cord of CCI rats. Mechanistic analysis unveiled that the inhibition of antioxidant pathway of Nrf2 signalling via its specific inhibitor, ML385, significantly countered EA's protective effect against neuronal ferroptosis in NP rats while marginally diminishing its analgesic effect. These findings suggest that EA treatment at acupoints ST36 and GV20 may protect against NP by inhibiting neuronal ferroptosis in the spinal cord, partially through the activation of Nrf2 signalling.
Keywords: electroacupuncture; ferroptosis; glutathione peroxidase 4; neuronal protection; neuropathic pain; oxidative stress.
© 2024 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
[Electroacupuncture improves ischemic myocardial injury by activating Nrf2/HO-1 signaling pathway to inhibit ferroptosis in rats].Zhen Ci Yan Jiu. 2023 May 25;48(5):461-7. doi: 10.13702/j.1000-0607.20211358. Zhen Ci Yan Jiu. 2023. PMID: 37247859 Chinese.
-
Electroacupuncture Ameliorates Chronic Inflammatory Pain and Depression Comorbidity by Inhibiting Nrf2-Mediated Ferroptosis in Hippocampal Neurons.Neurochem Res. 2025 Apr 21;50(3):149. doi: 10.1007/s11064-025-04401-2. Neurochem Res. 2025. PMID: 40257585
-
Electroacupuncture Downregulating Neuronal Ferroptosis in MCAO/R Rats by Activating Nrf2/SLC7A11/GPX4 Axis.Neurochem Res. 2024 Aug;49(8):2105-2119. doi: 10.1007/s11064-024-04185-x. Epub 2024 May 31. Neurochem Res. 2024. PMID: 38819696 Free PMC article.
-
Electroacupuncture suppresses glucose metabolism and GLUT-3 expression in medial prefrontal cortical in rats with neuropathic pain.Biol Res. 2021 Aug 6;54(1):24. doi: 10.1186/s40659-021-00348-0. Biol Res. 2021. PMID: 34362470 Free PMC article. Review.
-
The analgesic mechanism of electroacupuncture at the central level for neuropathic pain: a review of studies based on animal experiments.Front Neurol. 2025 May 29;16:1587471. doi: 10.3389/fneur.2025.1587471. eCollection 2025. Front Neurol. 2025. PMID: 40510205 Free PMC article. Review.
Cited by
-
Accurate Chemogenetics Determines Electroacupuncture Analgesia Through Increased CB1 to Suppress the TRPV1 Pathway in a Mouse Model of Fibromyalgia.Life (Basel). 2025 May 20;15(5):819. doi: 10.3390/life15050819. Life (Basel). 2025. PMID: 40430245 Free PMC article.
-
Electroacupuncture attenuates ferroptosis by promoting Nrf2 nuclear translocation and activating Nrf2/SLC7A11/GPX4 pathway in ischemic stroke.Chin Med. 2025 Jan 4;20(1):4. doi: 10.1186/s13020-024-01047-0. Chin Med. 2025. PMID: 39755657 Free PMC article.
-
The effect of acupuncture combined with hyperbaric oxygenation compared with hyperbaric oxygenation alone for patients with traumatic brain injury: a systematic review and meta-analysis.Front Neurol. 2025 May 2;16:1538740. doi: 10.3389/fneur.2025.1538740. eCollection 2025. Front Neurol. 2025. PMID: 40386021 Free PMC article.
-
Mechanisms and Therapeutic Potential of GPX4 in Pain Modulation.Pain Ther. 2025 Feb;14(1):21-45. doi: 10.1007/s40122-024-00673-8. Epub 2024 Nov 6. Pain Ther. 2025. PMID: 39503961 Free PMC article. Review.
-
Sustainable Release Selenium Laden with SiO2 Restoring Peripheral Nerve Injury via Modulating PI3K/AKT Pathway Signaling Pathway.Int J Nanomedicine. 2024 Aug 1;19:7851-7870. doi: 10.2147/IJN.S460397. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39105098 Free PMC article.
References
-
- Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101(1):259‐301. - PubMed
-
- Alles SRA, Smith PA. Etiology and pharmacology of neuropathic pain. Pharmacol Rev. 2018;70(2):315‐347. - PubMed
-
- Zhao XY, Zhang QS, Yang J, et al. The role of arginine vasopressin in electroacupuncture treatment of primary sciatica in human. Neuropeptides. 2015;52:61‐65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2020LK063/Shanghai University of Traditional Chinese Medicine
- TCM[2020]10/Shanghai University of Traditional Chinese Medicine
- TCM[2020]23/Shanghai University of Traditional Chinese Medicine
- WL-HBMS-2022003K/Shanghai Municipal Hospital of Traditional Chinese Medicine
- WL-HBMS-2022002K/Shanghai Municipal Hospital of Traditional Chinese Medicine
LinkOut - more resources
Full Text Sources
Miscellaneous

