Surveillance of COVID-19 vaccines: A comprehensive analysis of the first immunization drive in Ecuador
- PMID: 38509901
- PMCID: PMC10951513
- DOI: 10.1016/j.heliyon.2024.e27464
Surveillance of COVID-19 vaccines: A comprehensive analysis of the first immunization drive in Ecuador
Abstract
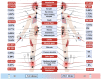
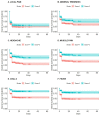
The initial phase of the COVID-19 vaccination in Ecuador occurred between April and November 2021. Initially, it focused on priority populations, including health professionals and other front-line workers. During this period, there was limited knowledge about the vaccine's adverse effects. A non-probability, observational study was conducted among university staff in Guayaquil, Ecuador, who received the AstraZeneca vaccine (n = 423) between April and November 2021. This study aimed to compare the acute adverse reactions by doses and to report the incidence of long-term adverse reactions within the AstraZeneca group. As a result, comparing acute adverse reactions between doses, the odds ratio for local pain, headache, muscle pain, fever, and chills are statistically higher after the first dose than the second dose. Survival curves indicated these symptoms appeared mainly within the first 6 h post-vaccination. This is the first pharmacovigilance study from Ecuador that analyzes survival probabilities for the AstraZeneca vaccine's adverse effects.
Keywords: AstraZeneca; COVID-19; Ecuador; Immunization; Vaccine; pharmacovigilance.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Regulation and Prequalification, (n.d.) https://www.who.int/teams/regulation-prequalification/regulation-and-saf... (accessed June 5, 2023).
-
- Olivera M.E., Uema S.A.N., Romañuk C.B., Caffaratti M., Mastroianni P.C., Varallo F.R., Vazquez M., Fagiolino P., Maldonado C., Vega E.M., Galvan Z.V., Maidanag M., Acostag P., Rivero R., Barros C., Fontana D. Regulatory issues on pharmacovigilance in Latin American countries. Currículo Lattes. 2014;16:289–312. doi: 10.3233/PPL-140390. - DOI
-
- Wang C., Zhu J., Zhang D. The paradox of information Control under authoritarianism: explaining trust in competing messages in China. Polit. Stud. 2023 doi: 10.1177/00323217231191399. - DOI
-
- Ecuador Receives its First COVID-19 Vaccines through the COVAX Facility - PAHO/WHO | Pan American Health Organization, (n.d.). https://www.paho.org/en/news/17-3-2021-ecuador-receives-its-first-covid-... June 5, 2023).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

