Exosomes promote pre-metastatic niche formation in colorectal cancer
- PMID: 38509970
- PMCID: PMC10950591
- DOI: 10.1016/j.heliyon.2024.e27572
Exosomes promote pre-metastatic niche formation in colorectal cancer
Abstract
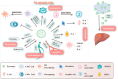
It is well known that colorectal cancer (CRC) has a high morbidity rate, a poor prognosis when metastasized, and a greatly shortened 5-year survival rate. Therefore, understanding the mechanism of tumor metastasis is still important. Based on the "seed and soil" theory, the concept of " premetastatic niche (PMN)" was introduced by Kaplan et al. The complex interaction between primary tumors and the metastatic organ provides a beneficial microenvironment for tumor cells to colonize at a distance. With further exploration of the PMN, exosomes have gradually attracted interest from researchers. Exosomes are extracellular vesicles secreted from cells that include various biological information and are involved in communication between cells. As a key molecule in the PMN, exosomes are closely related to tumor metastasis. In this article, we obtained information by conducting a comprehensive search across academic databases including PubMed and Web of Science using relevant keywords. Only recent, peer-reviewed articles published in the English language were considered for inclusion. This study aims to explore in depth how exosomes promote the formation of pre-metastatic microenvironment (PMN) in colorectal cancer and its related mechanisms.
Keywords: Colorectal cancer (CRC); Exosomes; Liver metastases; Premetastatic niche (PMN).
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Understanding pre-metastatic niche formation: implications for colorectal cancer liver metastasis.J Transl Med. 2025 Mar 17;23(1):340. doi: 10.1186/s12967-025-06328-2. J Transl Med. 2025. PMID: 40098140 Free PMC article. Review.
-
The cellular and molecular components involved in pre-metastatic niche formation in colorectal cancer liver metastasis.Expert Rev Gastroenterol Hepatol. 2021 Apr;15(4):389-399. doi: 10.1080/17474124.2021.1848543. Epub 2020 Nov 30. Expert Rev Gastroenterol Hepatol. 2021. PMID: 33174441 Review.
-
Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer.J Hematol Oncol. 2020 Nov 19;13(1):156. doi: 10.1186/s13045-020-00991-2. J Hematol Oncol. 2020. PMID: 33213490 Free PMC article.
-
Exosomes Promote Pre-Metastatic Niche Formation in Gastric Cancer.Front Oncol. 2021 May 24;11:652378. doi: 10.3389/fonc.2021.652378. eCollection 2021. Front Oncol. 2021. PMID: 34109113 Free PMC article. Review.
-
Exosomal proteins: Key players mediating pre‑metastatic niche formation and clinical implications (Review).Int J Oncol. 2021 Apr;58(4):4. doi: 10.3892/ijo.2021.5184. Epub 2021 Mar 2. Int J Oncol. 2021. PMID: 33649844 Free PMC article. Review.
Cited by
-
Exosomal ncRNAs in reproductive cancers†.Biol Reprod. 2025 Feb 14;112(2):225-244. doi: 10.1093/biolre/ioae170. Biol Reprod. 2025. PMID: 39561105 Free PMC article. Review.
-
Nanoparticles-Delivered Circular RNA Strategy as a Novel Antitumor Approach.Int J Mol Sci. 2024 Aug 16;25(16):8934. doi: 10.3390/ijms25168934. Int J Mol Sci. 2024. PMID: 39201617 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

