Effect of continuous renal replacement therapy on the clinical efficacy and pharmacokinetics of polymyxin B in the treatment of severe pulmonary infection
- PMID: 38509986
- PMCID: PMC10951545
- DOI: 10.1016/j.heliyon.2024.e27558
Effect of continuous renal replacement therapy on the clinical efficacy and pharmacokinetics of polymyxin B in the treatment of severe pulmonary infection
Abstract
Objective: This study aimed to evaluate the pharmacokinetics of polymyxin B in patients with ventilator-associated pneumonia caused by multi-drug resistant bacteria, and to analyze the effect of continuous renal replacement therapy (CRRT) on pharmacokinetics of polymyxin B.
Methods: Thirty-five patients with ventilator-associated pneumonia caused by multi-drug resistant bacteria admitted to our hospital from June 2021 to January 2022 were selected as the subjects. The patients were divided into the standard group (n = 20) and the non-standard group (n = 15) based on the factors affecting the compliance of polymyxin B plasma concentration. The patients received with polymyxin B and the plasma concentration was monitored. According to the monitoring results, they were divided into the standard group and the non-standard group, to analyze the influencing factors of polymyxin B on the blood concentration. Besides, the patients were then divided into the control group (n = 28) and the observation group (n = 7) according to whether the patients received CRRT treatment. Patients in the control group treated with polymyxin B alone, while patients in the observation group received with polymyxin B and CRRT. The general data of patients in the two groups were compared. The levels of plasma concentration of polymyxin B measured before the next administration (Cmin), peak plasma concentration of polymyxin B measured immediately after end of infusion (Cmax) and intermediate plasma concentration measured 6 h after administration (midpoint of the dosing interval) (C1/2t) were detected and compared between the two groups. Correlation between pharmacokinetics and efficacy was analyzed by Spearman correlation. The incidence of complications and the 28-day mortality rate of the two groups were recorded.
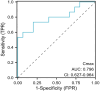
Results: The age, body mass index (BMI) and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores in the non-standard group were higher than these in the standard group (p < 0.05). BMI and APACHE II scores were independent risk factors affecting the pharmacokinetics of polymyxin B in patients with severe pulmonary infection (p < 0.05). There were no significant differences in age, BMI, APACHEII score, alanine aminotransferase level, aspartate aminotransferase level, albumin level, gender and diabetes ratio between the control group and the observation group (p > 0.05). The levels of Cmin, Cmax, and C1/2t in the observation group were lower than these in the control group (p < 0.001). The response rate was 50.00% in the control group and 36.36% in the observation group (p > 0.05). The levels of Cmin, Cmax, and C1/2t in the observation group were no significant correlation with the clinical efficacy (p > 0.05), while these in the control group were positive correlation with the clinical efficacy (r = 0.485, p < 0.05). There was no significant difference in the incidence of skin pigmentation, nephrotoxicity and 28-day mortality between the two groups (p > 0.05).
Conclusion: In patients with ventilator-associated pneumonia not receiving multidrug-resistant bacteria, the rate of achieving blood drug concentration with the usual recommended dose of polymyxin B was satisfactory. However, the proportion of patients with a 6-h plasma concentration exceeding the maximum plasma concentration was high. BMI and APACHE II scores were important factors affecting the pharmacokinetics of polymyxin B. In patients undergoing CRRT, the plasma concentration of polymyxin B was significantly reduced, suggesting that in patients with severe disease, plasma concentration monitoring played an important role in drug efficacy and patient safety. In patients treated with CRRT, the dose of polymyxin B may need to be increased.
Keywords: Clinical efficacy; Continuous renal replacement therapy; Effect; Plasma concentration; Polymyxin B; Severe pulmonary infection.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Mukhopadhyay S. Introduction to "pathology of infections of the lung". Semin. Diagn. Pathol. 2017;34(6):497. - PubMed
-
- Falagas M.E., Kyriakidou M., Voulgaris G.L., Vokos F., Politi S., Kechagias K.S. Clinical use of intravenous polymyxin B for the treatment of patients with multidrug-resistant Gram-negative bacterial infections: an evaluation of the current evidence. Journal of global antimicrobial resistance. 2021;24:342–359. - PubMed
LinkOut - more resources
Full Text Sources

