Non-random spatial organization of telomeres varies during the cell cycle and requires LAP2 and BAF
- PMID: 38510147
- PMCID: PMC10951912
- DOI: 10.1016/j.isci.2024.109343
Non-random spatial organization of telomeres varies during the cell cycle and requires LAP2 and BAF
Abstract
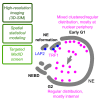
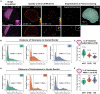
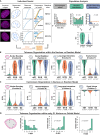
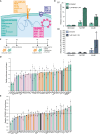
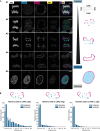
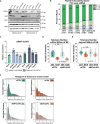
Spatial genome organization within the nucleus influences major biological processes and is impacted by the configuration of linear chromosomes. Here, we applied 3D spatial statistics and modeling on high-resolution telomere and centromere 3D-structured illumination microscopy images in cancer cells. We found a multi-scale organization of telomeres that dynamically evolved from a mixed clustered-and-regular distribution in early G1 to a purely regular distribution as cells progressed through the cell cycle. In parallel, our analysis revealed two pools of peripheral and internal telomeres, the proportions of which were inverted during the cell cycle. We then conducted a targeted screen using MadID to identify the molecular pathways driving or maintaining telomere anchoring to the nuclear envelope observed in early G1. Lamina-associated polypeptide (LAP) proteins were found transiently localized to telomeres in anaphase, a stage where LAP2α initiates the reformation of the nuclear envelope, and impacted telomere redistribution in the next interphase together with their partner barrier-to-autointegration factor (BAF).
Keywords: Chromosome organization; Membrane architecture; Molecular interaction.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
LAP2alpha and BAF transiently localize to telomeres and specific regions on chromatin during nuclear assembly.J Cell Sci. 2004 Dec 1;117(Pt 25):6117-28. doi: 10.1242/jcs.01529. Epub 2004 Nov 16. J Cell Sci. 2004. PMID: 15546916
-
Barrier-to-autointegration factor plays crucial roles in cell cycle progression and nuclear organization in Drosophila.J Cell Sci. 2003 Sep 15;116(Pt 18):3811-23. doi: 10.1242/jcs.00682. Epub 2003 Aug 5. J Cell Sci. 2003. PMID: 12902403
-
LEM4/ANKLE-2 deficiency impairs post-mitotic re-localization of BAF, LAP2α and LaminA to the nucleus, causes nuclear envelope instability in telophase and leads to hyperploidy in HeLa cells.Eur J Cell Biol. 2018 Jan;97(1):63-74. doi: 10.1016/j.ejcb.2017.12.001. Epub 2017 Dec 11. Eur J Cell Biol. 2018. PMID: 29254732
-
The nuclear lamina: molecular organization and interaction with chromatin.Crit Rev Eukaryot Gene Expr. 1999;9(3-4):285-93. doi: 10.1615/critreveukargeneexpr.v9.i3-4.130. Crit Rev Eukaryot Gene Expr. 1999. PMID: 10651245 Review.
-
Review: lamina-associated polypeptide 2 isoforms and related proteins in cell cycle-dependent nuclear structure dynamics.J Struct Biol. 2000 Apr;129(2-3):335-45. doi: 10.1006/jsbi.2000.4212. J Struct Biol. 2000. PMID: 10806084 Review.
Cited by
-
Preserving Genome Integrity: Unveiling the Roles of ESCRT Machinery.Cells. 2024 Aug 5;13(15):1307. doi: 10.3390/cells13151307. Cells. 2024. PMID: 39120335 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

