Exogenous IL-2 delays memory precursors generation and is essential for enhancing memory cells effector functions
- PMID: 38510150
- PMCID: PMC10952031
- DOI: 10.1016/j.isci.2024.109411
Exogenous IL-2 delays memory precursors generation and is essential for enhancing memory cells effector functions
Abstract
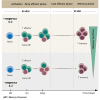
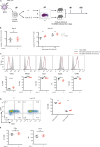
To investigate the impact of paracrine IL-2 signals on memory precursor (MP) cell differentiation, we activated CD8 T cell in vitro in the presence or absence of exogenous IL-2 (ex-IL-2). We assessed memory differentiation by transferring these cells into virus-infected mice. Both conditions generated CD8 T cells that participate in the ongoing response and gave rise to similar memory cells. Nevertheless, when transferred into a naive host, T cells activated with ex-IL-2 generated a higher frequency of memory cells displaying increased functional memory traits. Single-cell RNA-seq analysis indicated that without ex-IL-2, cells rapidly acquire an MP signature, while in its presence they adopted an effector signature. This was confirmed at the protein level and in a functional assay. Overall, ex-IL-2 delays the transition into MP cells, allowing the acquisition of effector functions that become imprinted in their progeny. These findings may help to optimize the generation of therapeutic T cells.
Keywords: Biological sciences; Immune system evolution; Immunology; Molecular biology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Kahan S.M., Bakshi R.K., Ingram J.T., Hendrickson R.C., Lefkowitz E.J., Crossman D.K., Harrington L.E., Weaver C.T., Zajac A.J. Intrinsic IL-2 production by effector CD8 T cells affects IL-2 signaling and promotes fate decisions, stemness, and protection. Sci. Immunol. 2022;7 doi: 10.1126/sciimmunol.abl6322. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

