Linking sarcopenia, brain structure and cognitive performance: a large-scale UK Biobank study
- PMID: 38510210
- PMCID: PMC10953622
- DOI: 10.1093/braincomms/fcae083
Linking sarcopenia, brain structure and cognitive performance: a large-scale UK Biobank study
Abstract
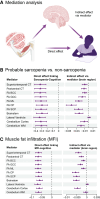
Sarcopenia refers to age-related loss of muscle mass and function and is related to impaired somatic and brain health, including cognitive decline and Alzheimer's disease. However, the relationships between sarcopenia, brain structure and cognition are poorly understood. Here, we investigate the associations between sarcopenic traits, brain structure and cognitive performance. We included 33 709 UK Biobank participants (54.2% female; age range 44-82 years) with structural and diffusion magnetic resonance imaging, thigh muscle fat infiltration (n = 30 561) from whole-body magnetic resonance imaging (muscle quality indicator) and general cognitive performance as indicated by the first principal component of a principal component analysis across multiple cognitive tests (n = 22 530). Of these, 1703 participants qualified for probable sarcopenia based on low handgrip strength, and we assigned the remaining 32 006 participants to the non-sarcopenia group. We used multiple linear regression to test how sarcopenic traits (probable sarcopenia versus non-sarcopenia and percentage of thigh muscle fat infiltration) relate to cognitive performance and brain structure (cortical thickness and area, white matter fractional anisotropy and deep and lower brain volumes). Next, we used structural equation modelling to test whether brain structure mediated the association between sarcopenic and cognitive traits. We adjusted all statistical analyses for confounders. We show that sarcopenic traits (probable sarcopenia versus non-sarcopenia and muscle fat infiltration) are significantly associated with lower cognitive performance and various brain magnetic resonance imaging measures. In probable sarcopenia, for the included brain regions, we observed widespread significant lower white matter fractional anisotropy (77.1% of tracts), predominantly lower regional brain volumes (61.3% of volumes) and thinner cortical thickness (37.9% of parcellations), with |r| effect sizes in (0.02, 0.06) and P-values in (0.0002, 4.2e-29). In contrast, we observed significant associations between higher muscle fat infiltration and widespread thinner cortical thickness (76.5% of parcellations), lower white matter fractional anisotropy (62.5% of tracts) and predominantly lower brain volumes (35.5% of volumes), with |r| effect sizes in (0.02, 0.07) and P-values in (0.0002, 1.9e-31). The regions showing the most significant effect sizes across the cortex, white matter and volumes were of the sensorimotor system. Structural equation modelling analysis revealed that sensorimotor brain regions mediate the link between sarcopenic and cognitive traits [probable sarcopenia: P-values in (0.0001, 1.0e-11); muscle fat infiltration: P-values in (7.7e-05, 1.7e-12)]. Our findings show significant associations between sarcopenic traits, brain structure and cognitive performance in a middle-aged and older adult population. Mediation analyses suggest that regional brain structure mediates the association between sarcopenic and cognitive traits, with potential implications for dementia development and prevention.
Keywords: T1-weighted MRI; degenerative conditions; ectopic fat; mediator; skeletal muscle.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
O.A.A. has received a speaker's honorarium from Lundbeck, Sunovion, Otsuka and Janssen and is a consultant to Cortechs.ai. J.L. is an employee and shareholder of AMRA Medical AB and has received consulting honorarium/speaking fees from Eli Lilly and BioMarin. The remaining authors declare no conflict of interest.
Figures



References
-
- Yuan S, Larsson SC. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism. 2023;144:155533. - PubMed
-
- Dost FS, Ates Bulut E, Dokuzlar O, et al. Sarcopenia is as common in older patients with dementia with Lewy bodies as it is in those with Alzheimer's disease. Geriatr Gerontol Int. 2022;22(5):418–424. - PubMed
-
- Waite SJ, Maitland S, Thomas A, Yarnall AJ. Sarcopenia and frailty in individuals with dementia: A systematic review. Arch Gerontol Geriatr. 2021;92:104268. - PubMed
LinkOut - more resources
Full Text Sources
