Cerebellar degeneration in gluten ataxia is linked to microglial activation
- PMID: 38510211
- PMCID: PMC10953628
- DOI: 10.1093/braincomms/fcae078
Cerebellar degeneration in gluten ataxia is linked to microglial activation
Abstract
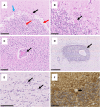
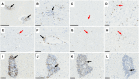
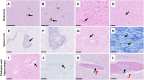
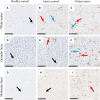
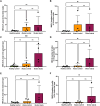
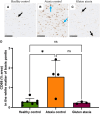
Gluten sensitivity has long been recognized exclusively for its gastrointestinal involvement; however, more recent research provides evidence for the existence of neurological manifestations that can appear in combination with or independent of the small bowel manifestations. Amongst all neurological manifestations of gluten sensitivity, gluten ataxia is the most commonly occurring one, accounting for up to 40% of cases of idiopathic sporadic ataxia. However, despite its prevalence, its neuropathological basis is still poorly defined. Here, we provide a neuropathological characterization of gluten ataxia and compare the presence of neuroinflammatory markers glial fibrillary acidic protein, ionized calcium-binding adaptor molecule 1, major histocompatibility complex II and cluster of differentiation 68 in the central nervous system of four gluten ataxia cases to five ataxia controls and seven neurologically healthy controls. Our results demonstrate that severe cerebellar atrophy, cluster of differentiation 20+ and cluster of differentiation 8+ lymphocytic infiltration in the cerebellar grey and white matter and a significant upregulation of microglial immune activation in the cerebellar granular layer, molecular layer and cerebellar white matter are features of gluten ataxia, providing evidence for the involvement of both cellular and humoral immune-mediated processes in gluten ataxia pathogenesis.
Keywords: MHC-II; gluten ataxia; gluten sensitivity; neuroimmune responses; neuroinflammation.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
D.A. is a scientific advisor to Zedira and receives royalties for patents licensed to Zedira. The other authors report no competing interests.
Figures

Similar articles
-
Gluten ataxia in perspective: epidemiology, genetic susceptibility and clinical characteristics.Brain. 2003 Mar;126(Pt 3):685-91. doi: 10.1093/brain/awg050. Brain. 2003. PMID: 12566288
-
Gluten sensitivity in Japanese patients with adult-onset cerebellar ataxia.Intern Med. 2006;45(3):135-40. doi: 10.2169/internalmedicine.45.1351. Epub 2006 Mar 1. Intern Med. 2006. PMID: 16508226
-
Clinical, radiological, neurophysiological, and neuropathological characteristics of gluten ataxia.Lancet. 1998 Nov 14;352(9140):1582-5. doi: 10.1016/s0140-6736(98)05342-2. Lancet. 1998. PMID: 9843103
-
Sporadic cerebellar ataxia associated with gluten sensitivity.Brain. 2001 May;124(Pt 5):1013-9. doi: 10.1093/brain/124.5.1013. Brain. 2001. PMID: 11335703 Review.
-
Gluten-related disorders: gluten ataxia.Dig Dis. 2015;33(2):264-268. doi: 10.1159/000369509. Epub 2015 Apr 22. Dig Dis. 2015. PMID: 25925933 Review.
Cited by
-
CSF Markers of TG6 Autoimmunity in Gluten Ataxia.Cerebellum. 2025 Apr 2;24(3):79. doi: 10.1007/s12311-025-01832-z. Cerebellum. 2025. PMID: 40172737 Free PMC article.
References
-
- Hadjivassiliou M, Chattopadhyay AK, Grünewald RA, et al. Myopathy associated with gluten sensitivity. Muscle Nerve. 2007;35(4):443–450. - PubMed
-
- Hadjivassiliou M, Sanders DS, Grünewald RA, Woodroofe N, Boscolo S, Aeschlimann D. Gluten sensitivity: From gut to brain. Lancet Neurol. 2010;9(3):318–330. - PubMed
-
- Kieslich M, Errázuriz G, Posselt HG, Moeller-Hartmann W, Zanella F, Boehles H. Brain white-matter lesions in celiac disease: A prospective study of 75 diet-treated patients. Pediatrics. 2001;108(2):e21. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
