In vivo PET classification of tau pathologies in patients with frontotemporal dementia
- PMID: 38510212
- PMCID: PMC10953627
- DOI: 10.1093/braincomms/fcae075
In vivo PET classification of tau pathologies in patients with frontotemporal dementia
Abstract
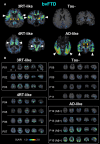
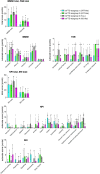
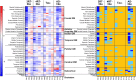
Frontotemporal dementia refers to a group of neurodegenerative disorders with diverse clinical and neuropathological features. In vivo neuropathological assessments of frontotemporal dementia at an individual level have hitherto not been successful. In this study, we aim to classify patients with frontotemporal dementia based on topologies of tau protein aggregates captured by PET with 18F-florzolotau (aka 18F-APN-1607 and 18F-PM-PBB3), which allows high-contrast imaging of diverse tau fibrils in Alzheimer's disease as well as in non-Alzheimer's disease tauopathies. Twenty-six patients with frontotemporal dementia, 15 with behavioural variant frontotemporal dementia and 11 with other frontotemporal dementia phenotypes, and 20 age- and sex-matched healthy controls were included in this study. They underwent PET imaging of amyloid and tau depositions with 11C-PiB and 18F-florzolotau, respectively. By combining visual and quantitative analyses of PET images, the patients with behavioural variant frontotemporal dementia were classified into the following subgroups: (i) predominant tau accumulations in frontotemporal and frontolimbic cortices resembling three-repeat tauopathies (n = 3), (ii) predominant tau accumulations in posterior cortical and subcortical structures indicative of four-repeat tauopathies (n = 4); (iii) amyloid and tau accumulations consistent with Alzheimer's disease (n = 4); and (iv) no overt amyloid and tau pathologies (n = 4). Despite these distinctions, clinical symptoms and localizations of brain atrophy did not significantly differ among the identified behavioural variant frontotemporal dementia subgroups. The patients with other frontotemporal dementia phenotypes were also classified into similar subgroups. The results suggest that PET with 18F-florzolotau potentially allows the classification of each individual with frontotemporal dementia on a neuropathological basis, which might not be possible by symptomatic and volumetric assessments.
Keywords: PET; biomarker; florzolotau; frontotemporal lobar degeneration; tauopathy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
H.Shimada, M.-R.Z. and M.H. hold patents on compounds related to the present report (JP 5422782/EP 12 884 742.3/CA2894994/HK1208672). All authors declare no competing interests.
Figures





Similar articles
-
An optimized reference tissue method for quantification of tau protein depositions in diverse neurodegenerative disorders by PET with 18F-PM-PBB3 (18F-APN-1607).Neuroimage. 2022 Dec 1;264:119763. doi: 10.1016/j.neuroimage.2022.119763. Epub 2022 Nov 24. Neuroimage. 2022. PMID: 36427751
-
High-Contrast In Vivo Imaging of Tau Pathologies in Alzheimer's and Non-Alzheimer's Disease Tauopathies.Neuron. 2021 Jan 6;109(1):42-58.e8. doi: 10.1016/j.neuron.2020.09.042. Epub 2020 Oct 29. Neuron. 2021. PMID: 33125873
-
Evaluation of [18F]PI-2620, a second-generation selective tau tracer, for assessing four-repeat tauopathies.Brain Commun. 2021 Aug 24;3(4):fcab190. doi: 10.1093/braincomms/fcab190. eCollection 2021. Brain Commun. 2021. PMID: 34632382 Free PMC article.
-
Association Between Globular Glial Tauopathies and Frontotemporal Dementia-Expanding the Spectrum of Gliocentric Disorders: A Review.JAMA Neurol. 2021 Aug 1;78(8):1004-1014. doi: 10.1001/jamaneurol.2021.1813. JAMA Neurol. 2021. PMID: 34152367 Review.
-
Frontotemporal lobar degeneration. An update on clinical, pathological and genetic findings.Gerontology. 2001 Jan-Feb;47(1):1-8. doi: 10.1159/000052763. Gerontology. 2001. PMID: 11244285 Review.
Cited by
-
Neuropathological correlations of 18F-florzolotau PET in a case with pick's disease.EJNMMI Res. 2025 Jul 31;15(1):96. doi: 10.1186/s13550-025-01296-6. EJNMMI Res. 2025. PMID: 40745396 Free PMC article.
-
Diverse tau pathologies in late-life mood disorders revealed by PET and autopsy assays.Alzheimers Dement. 2025 Jun;21(6):e70195. doi: 10.1002/alz.70195. Alzheimers Dement. 2025. PMID: 40488253 Free PMC article.
References
-
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
