Cardiac Troponin in Patients With Light Chain and Transthyretin Cardiac Amyloidosis: JACC: CardioOncology State-of-the-Art Review
- PMID: 38510286
- PMCID: PMC10950441
- DOI: 10.1016/j.jaccao.2023.12.006
Cardiac Troponin in Patients With Light Chain and Transthyretin Cardiac Amyloidosis: JACC: CardioOncology State-of-the-Art Review
Abstract
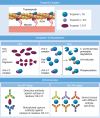
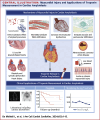
Cardiac amyloidosis (CA) is an infiltrative disease caused by amyloid fibril deposition in the myocardium; the 2 forms that most frequently involve the heart are amyloid light chain (AL) and amyloid transthyretin (ATTR) amyloidosis. Cardiac troponin (cTn) is the biomarker of choice for the detection of myocardial injury and is frequently found to be elevated in patients with CA, particularly with high-sensitivity assays. Multiple mechanisms of myocardial injury in CA have been proposed, including cytotoxic effect of amyloid precursors, interstitial amyloid fibril infiltration, coronary microvascular dysfunction, amyloid- and non-amyloid-related coronary artery disease, diastolic dysfunction, and heart failure. Regardless of the mechanisms, cTn values have relevant prognostic (and potentially diagnostic) implications in both AL and ATTR amyloidosis. In this review, the authors discuss the significant aspects of cTn biology and measurement methods, potential mechanisms of myocardial injury in CA, and the clinical application of cTn in the management of both AL and ATTR amyloidosis.
Keywords: amyloidosis; diagnosis; myocardial injury; prognosis; troponin.
© 2024 The Authors.
Conflict of interest statement
Dr De Michieli has received honoraria from Pfizer, Alnylam Pharmaceuticals, and AstraZeneca. Dr Cipriani has received honoraria from Pfizer, Alnylam Pharmaceuticals, and AstraZeneca. Dr Dispenzieri has received research support from Alnylam Pharmaceuticals, Pfizer, Takeda, and Bristol Myers Squibb; participates on the data and safety monitoring board for Oncopeptides and Sorrento; and is on the advisory board and independent review committee for Janssen. Dr Jaffe has consulted or presently consults for most of the major diagnostics companies, including Beckman-Coulter, Abbott, Siemens, Ortho Diagnostics, ET Healthcare, Roche, Radiometer, SphingoTec, Amgen, and Novartis; and he has stock options in RCE Technologies.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

