Impact of the ESC Cardio-Oncology Guidelines Biomarker Criteria on Incidence of Cancer Therapy-Related Cardiac Dysfunction
- PMID: 38510299
- PMCID: PMC10950440
- DOI: 10.1016/j.jaccao.2023.10.008
Impact of the ESC Cardio-Oncology Guidelines Biomarker Criteria on Incidence of Cancer Therapy-Related Cardiac Dysfunction
Abstract
Background: The impact of recent consensus definitions of cancer therapy-related cardiac dysfunction (CTRCD) from the European Society of Cardiology cardio-oncology guidelines on the reported incidence of CTRCD has not yet been assessed.
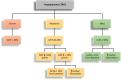
Objectives: The aim of this study was to assess the: 1) cumulative incidence; 2) point prevalence during and after adjuvant therapy; and 3) prognostic value of CTRCD as defined by different asymptomatic CTRCD guideline criteria.
Methods: The cumulative incidence and point prevalence of CTRCD were retrospectively assessed in 118 patients participating in the PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) trial. Asymptomatic CTRCD was assessed using alternative cardiac troponin (cTn) 99th percentile upper reference limits (URLs) to define cTnT and cTnI elevation.
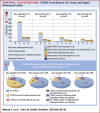
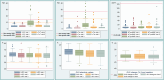
Results: The cumulative incidence of moderate or severe CTRCD was low (1.7%), whereas the cumulative incidence of mild asymptomatic CTRCD was higher and differed markedly according to the biomarker criteria applied, ranging from 49.2% of patients when cTnT greater than the sex-specific 99th percentile URL was used to define cTn elevation to 9.3% when sex-neutral cTnI was used. The point prevalence of CTRCD was highest at the end of anthracycline therapy (47.8%) and was driven primarily by asymptomatic cTn elevation. CTRCD during adjuvant therapy was not prognostic for CTRCD at extended follow-up of 24 months (Q1-Q3: 21-29 months) after randomization.
Conclusions: Mild asymptomatic CTRCD during adjuvant breast cancer therapy was frequent and driven mainly by cTn elevation and was not prognostic of subsequent CTRCD. The incidence of mild, asymptomatic CTRCD differed markedly depending on the cTn assay and whether sex-neutral or sex-dependent URLs were applied. (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy [PRADA]; NCT01434134).
Keywords: biomarkers; breast cancer; cardiac magnetic resonance; cardiomyopathy; guidelines; troponin.
© 2024 The Authors.
Conflict of interest statement
This work was supported by the South-Eastern Norway Regional Health Authority, the University of Oslo, the Extra Foundation for Health and Rehabilitation, Norway, the Norwegian Cancer Society, and Akershus University Hospital. Study medications and matching placebos were provided free of charge by AstraZeneca. Reagents for the analysis of high-sensitivity cTnI were provided by Abbott Diagnostics. Dr Gulati has received speaker honoraria from Novartis, AstraZeneca, and Bristol Myers Squibb. Dr Omland has served on advisory boards for Abbott Diagnostics, Roche Diagnostics, and Bayer; has received research support from Abbott Diagnostics, Novartis, and Roche Diagnostics via Akershus University Hospital; and has received speaker or consulting honoraria from Roche Diagnostics, Siemens Healthineers, and CardiNor. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
-
- Lyon A.R., Lopez-Fernandez T., Couch L.S., et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43(41):4229–4361. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

