A comprehensive review of monkeypox virus and mpox characteristics
- PMID: 38510963
- PMCID: PMC10952103
- DOI: 10.3389/fcimb.2024.1360586
A comprehensive review of monkeypox virus and mpox characteristics
Abstract
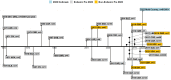
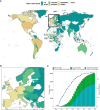
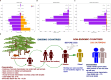
Monkeypox virus (MPXV) is the etiological agent of monkeypox (mpox), a zoonotic disease. MPXV is endemic in the forested regions of West and Central Africa, but the virus has recently spread globally, causing outbreaks in multiple non-endemic countries. In this paper, we review the characteristics of the virus, including its ecology, genomics, infection biology, and evolution. We estimate by phylogenomic molecular clock that the B.1 lineage responsible for the 2022 mpox outbreaks has been in circulation since 2016. We interrogate the host-virus interactions that modulate the virus infection biology, signal transduction, pathogenesis, and host immune responses. We highlight the changing pathophysiology and epidemiology of MPXV and summarize recent advances in the prevention and treatment of mpox. In addition, this review identifies knowledge gaps with respect to the virus and the disease, suggests future research directions to address the knowledge gaps, and proposes a One Health approach as an effective strategy to prevent current and future epidemics of mpox.
Keywords: antivirals; biosafety; epidemiology; evolution; genomics; infection biology; monkeypox; one health.
Copyright © 2024 Alakunle, Kolawole, Diaz-Cánova, Alele, Adegboye, Moens and Okeke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Aden T.A., Blevins P., York S.W., Rager S., Balachandran D., Huston C.L., et al. (2022). Rapid diagnostic testing for response to the monkeypox outbreak — Laboratory response network, United States, may 17–june 30, 2022. MMWR Morb. Mortal Wkly. Rep. 71, 904–907. doi: 10.15585/mmwr.mm7128e1 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

