Steroid-free combination of 5-azacytidine and venetoclax for the treatment of multiple myeloma
- PMID: 38511268
- PMCID: PMC11367189
- DOI: 10.3324/haematol.2023.283771
Steroid-free combination of 5-azacytidine and venetoclax for the treatment of multiple myeloma
Abstract
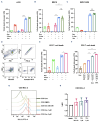
Multiple myeloma (MM) is an incurable plasma cell malignancy that, despite an unprecedented increase in overall survival, lacks truly risk-adapted or targeted treatments. A proportion of patients with MM depend on BCL-2 for survival, and, recently, the BCL-2 antagonist venetoclax has shown clinical efficacy and safety in t(11;14) and BCL-2 overexpressing MM. However, only a small proportion of MM patients rely on BCL-2 (approx. 20%), and there is a need to broaden the patient population outside of t(11;14) that can be treated with venetoclax. Therefore, we took an unbiased screening approach and screened epigenetic modifiers to enhance venetoclax sensitivity in 2 non-BCL-2 dependent MM cell lines. The demethylase inhibitor 5-azacytidine was one of the lead hits from the screen, and the enhanced cell killing of the combination was confirmed in additional MM cell lines. Using dynamic BH3 profiling and immunoprecipitations, we identified the potential mechanism of synergy is due to increased NOXA expression, through the integrated stress response. Knockdown of PMAIP1 or PKR partially rescues cell death of the venetoclax and 5-azacytidine combination treatment. The addition of a steroid to the combination treatment did not enhance the cell death, and, interestingly, we found enhanced death of the immune cells with steroid addition, suggesting that a steroid-sparing regimen may be more beneficial in MM. Lastly, we show for the first time in primary MM patient samples that 5-azacytidine enhances the response to venetoclax ex vivo across diverse anti-apoptotic dependencies (BCL-2 or MCL-1) and diverse cytogenetic backgrounds. Overall, our data identify 5-azacytidine and venetoclax as an effective treatment combination, which could be a tolerable steroid-sparing regimen, particularly for elderly MM patients.
Figures





References
-
- Cancer Factsheet Multiple Myeloma (ICD-10 C90) NCRI. https://www.ncri.ie/factsheets Accessed June 28, 2023.
-
- Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(5):548-567. - PubMed
-
- Landgren O, Weiss BM. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic / racial groups: support for genetic factors in pathogenesis. Leukemia. 2009;23(10):1691-1697. - PubMed
-
- Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125(20):3076-3084. - PubMed
-
- Maji S, Panda S, Samal SK, et al. . Bcl-2 antiapoptotic family proteins and chemoresistance in cancer. Adv Cancer Res. 2018;137:37-75. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

