Positioning centrioles and centrosomes
- PMID: 38512059
- PMCID: PMC10959756
- DOI: 10.1083/jcb.202311140
Positioning centrioles and centrosomes
Abstract
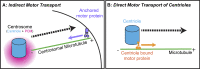
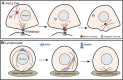
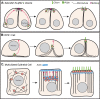
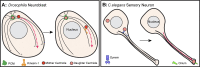
Centrosomes are the primary microtubule organizer in eukaryotic cells. In addition to shaping the intracellular microtubule network and the mitotic spindle, centrosomes are responsible for positioning cilia and flagella. To fulfill these diverse functions, centrosomes must be properly located within cells, which requires that they undergo intracellular transport. Importantly, centrosome mispositioning has been linked to ciliopathies, cancer, and infertility. The mechanisms by which centrosomes migrate are diverse and context dependent. In many cells, centrosomes move via indirect motor transport, whereby centrosomal microtubules engage anchored motor proteins that exert forces on those microtubules, resulting in centrosome movement. However, in some cases, centrosomes move via direct motor transport, whereby the centrosome or centriole functions as cargo that directly binds molecular motors which then walk on stationary microtubules. In this review, we summarize the mechanisms of centrosome motility and the consequences of centrosome mispositioning and identify key questions that remain to be addressed.
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures
Similar articles
-
Separate to operate: control of centrosome positioning and separation.Philos Trans R Soc Lond B Biol Sci. 2014 Sep 5;369(1650):20130461. doi: 10.1098/rstb.2013.0461. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25047615 Free PMC article. Review.
-
Common themes in centriole and centrosome movements.Trends Cell Biol. 2011 Jan;21(1):57-66. doi: 10.1016/j.tcb.2010.09.004. Epub 2010 Oct 18. Trends Cell Biol. 2011. PMID: 20961761 Review.
-
The Centrioles, Centrosomes, Basal Bodies, and Cilia of Drosophila melanogaster.Genetics. 2017 May;206(1):33-53. doi: 10.1534/genetics.116.198168. Genetics. 2017. PMID: 28476861 Free PMC article. Review.
-
Centrioles: active players or passengers during mitosis?Cell Mol Life Sci. 2010 Jul;67(13):2173-94. doi: 10.1007/s00018-010-0323-9. Epub 2010 Mar 19. Cell Mol Life Sci. 2010. PMID: 20300952 Free PMC article. Review.
-
The centrosomal linker and microtubules provide dual levels of spatial coordination of centrosomes.PLoS Genet. 2015 May 22;11(5):e1005243. doi: 10.1371/journal.pgen.1005243. eCollection 2015 May. PLoS Genet. 2015. PMID: 26001056 Free PMC article.
Cited by
-
Asymmetry of centrosomes in Drosophila neural stem cells requires protein phosphatase 4.Mol Biol Cell. 2025 May 1;36(5):ar58. doi: 10.1091/mbc.E25-01-0021. Epub 2025 Mar 12. Mol Biol Cell. 2025. PMID: 40072519
-
Microtubule-driven cell shape changes and actomyosin flow synergize to position the centrosome.J Cell Biol. 2025 Jul 7;224(7):e202405126. doi: 10.1083/jcb.202405126. Epub 2025 Apr 17. J Cell Biol. 2025. PMID: 40243666
-
eIF2A regulates cell migration in a translation-independent manner.Sci Adv. 2025 Aug;11(31):eadu5668. doi: 10.1126/sciadv.adu5668. Epub 2025 Aug 1. Sci Adv. 2025. PMID: 40749049 Free PMC article.
-
Axonemal Dynein Visualized in Primary Cilia via Expansion Microscopy.Cytoskeleton (Hoboken). 2025 Jun 24:10.1002/cm.70004. doi: 10.1002/cm.70004. Online ahead of print. Cytoskeleton (Hoboken). 2025. PMID: 40552592
-
Conservation of OFD1 Protein Motifs: Implications for Discovery of Novel Interactors and the OFD1 Function.Int J Mol Sci. 2025 Jan 29;26(3):1167. doi: 10.3390/ijms26031167. Int J Mol Sci. 2025. PMID: 39940934 Free PMC article.
References
-
- Aljiboury, A.A., Ingram E., Krishnan N., Ononiwu F., Pal D., Manikas J., Taveras C., Hall N.A., Da Silva J., Freshour J., and Hehnly H.. 2023. Rab8, Rab11, and Rab35 coordinate lumen and cilia formation during zebrafish left-right organizer development. PLoS Genet. 19:e1010765. 10.1371/journal.pgen.1010765 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

