Cascade Aryne Aminoarylation for Biaryl Phenol Synthesis
- PMID: 38512156
- PMCID: PMC11002935
- DOI: 10.1021/acs.orglett.4c00624
Cascade Aryne Aminoarylation for Biaryl Phenol Synthesis
Abstract
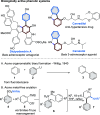
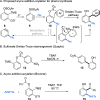
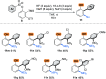
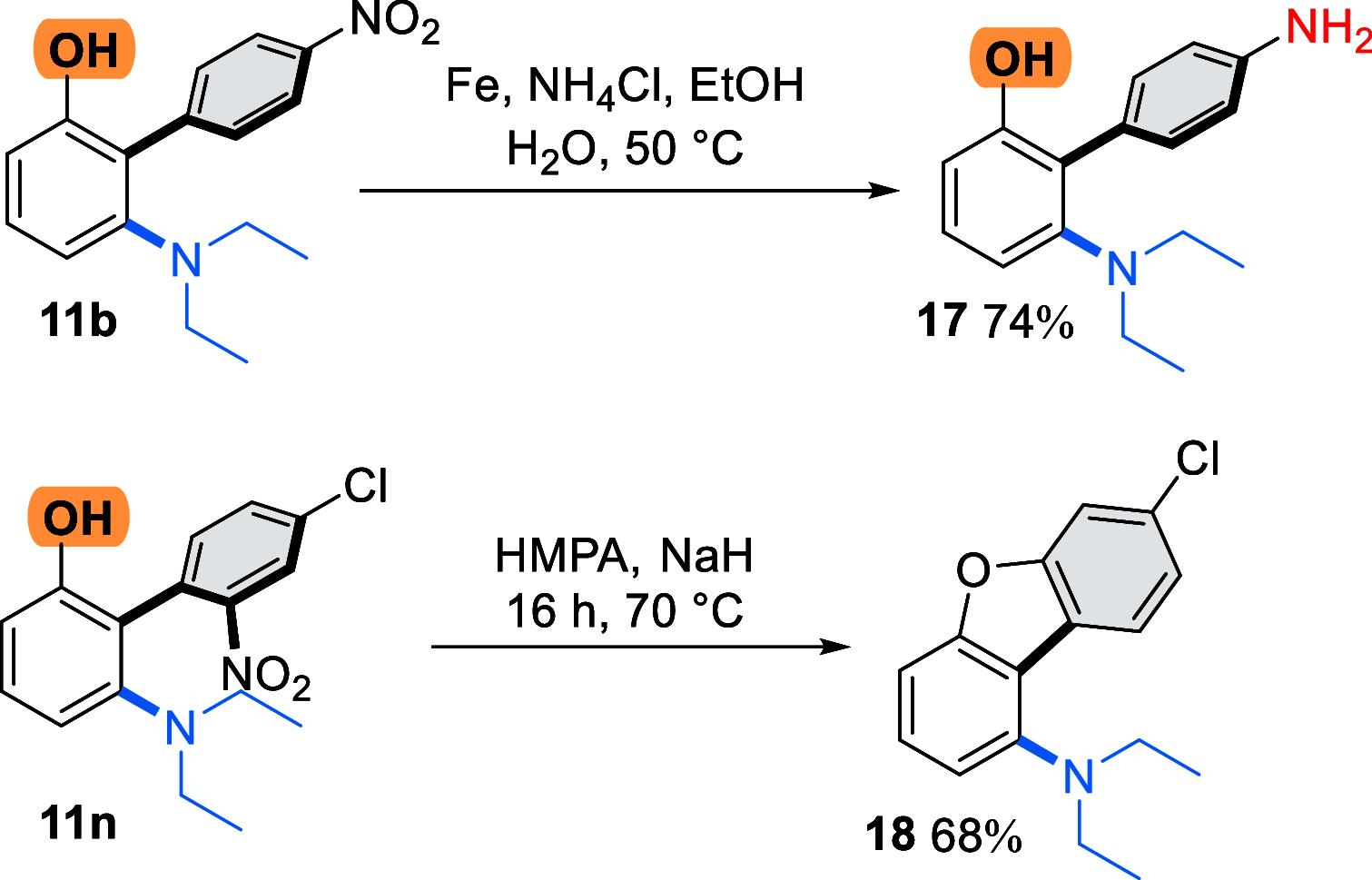
We describe a transition metal-free approach to hindered 3-amino-2-aryl phenols through a cascade nucleophilic addition / Smiles-Truce rearrangement of a functionalized Kobayashi aryne precursor. Under anionic conditions, secondary alkyl amines add to the aryne intermediate to set up an aryl transfer from a neighboring sulfonate group. The use of a sulfonate, rather than the more typical sulfonamide, enables access to phenolic biaryl products that are important motifs in natural products and pharmaceuticals.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
-
Recent benzyne reviews:
- Fluegel L. L.; Hoye T. R. Hexadehydro-Diels–Alder Reaction: Benzyne Generation via Cycloisomerization of Tethered Triynes. Chem. Rev. 2021, 121, 2413–2444. 10.1021/acs.chemrev.0c00825. - DOI - PMC - PubMed
- Roy T.; Biju A. T. Recent Advances in Molecular Rearrangements Involving Aryne Intermediates. Chem. Commun. 2018, 54, 2580–2594. 10.1039/C7CC09122B. - DOI - PubMed
- Matsuzawa T.; Yoshida S.; Hosoya T. Recent Advances in Reactions between Arynes and Organosulfur Compounds. Tetrahedron Lett. 2018, 59, 4197–4208. 10.1016/j.tetlet.2018.10.031. - DOI
- Takikawa H.; Nishii A.; Sakai T.; Suzuki K. Aryne-Based Strategy in the Total Synthesis of Naturally Occurring Polycyclic Compounds. Chem. Soc. Rev. 2018, 47, 8030–8056. 10.1039/C8CS00350E. - DOI - PubMed
-
-
- Wittig G. Phenyl-lithium, der Schlüssel zu einer neuen Chemie metallorganischer Verbindungen. Naturwissenschaften 1942, 30, 696–703. 10.1007/BF01489519. - DOI
-
- Himeshima Y.; Sonoda T.; Kobayashi H. Fluoride-Induces 1,2-Elimination of O-Trimethylsilylphenyl Triflate to Benzyne under Mild Conditions. Chem. Lett. 1983, 12, 1211–1214. 10.1246/cl.1983.1211. - DOI
-
Review:
- Shi J.; Li L.; Li Y. o-Silylaryl Triflates: A Journey of Kobayashi Aryne Precursors. Chem. Rev. 2021, 121, 3892–4044. 10.1021/acs.chemrev.0c01011. - DOI - PubMed
LinkOut - more resources
Full Text Sources