Vibration-Controlled Transient Elastography Scores to Predict Liver-Related Events in Steatotic Liver Disease
- PMID: 38512249
- PMCID: PMC10958386
- DOI: 10.1001/jama.2024.1447
Vibration-Controlled Transient Elastography Scores to Predict Liver-Related Events in Steatotic Liver Disease
Abstract
Importance: Metabolic dysfunction-associated steatotic liver disease (MASLD) is currently the most common chronic liver disease worldwide. It is important to develop noninvasive tests to assess the disease severity and prognosis.
Objective: To study the prognostic implications of baseline levels and dynamic changes of the vibration-controlled transient elastography (VCTE)-based scores developed for the diagnosis of advanced fibrosis (Agile 3+) and cirrhosis (Agile 4) in patients with MASLD.
Design, setting, and participants: This cohort study included data from a natural history cohort of patients with MASLD who underwent VCTE examination at 16 tertiary referral centers in the US, Europe, and Asia from February 2004 to January 2023, of which the data were collected prospectively at 14 centers. Eligible patients were adults aged at least 18 years with hepatic steatosis diagnosed by histologic methods (steatosis in ≥5% of hepatocytes) or imaging studies (ultrasonography, computed tomography or magnetic resonance imaging, or controlled attenuation parameter ≥248 dB/m by VCTE).
Main outcomes and measures: The primary outcome was liver-related events (LREs), defined as hepatocellular carcinoma or hepatic decompensation (ascites, variceal hemorrhage, hepatic encephalopathy, or hepatorenal syndrome), liver transplant, and liver-related deaths. The Agile scores were compared with histologic and 8 other noninvasive tests.
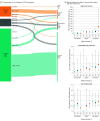
Results: A total of 16 603 patients underwent VCTE examination at baseline (mean [SD] age, 52.5 [13.7] years; 9600 [57.8%] were male). At a median follow-up of 51.7 (IQR, 25.2-85.2) months, 316 patients (1.9%) developed LREs. Both Agile 3+ and Agile 4 scores classified fewer patients between the low and high cutoffs than most fibrosis scores and achieved the highest discriminatory power in predicting LREs (integrated area under the time-dependent receiver-operating characteristic curve, 0.89). A total of 10 920 patients (65.8%) had repeated VCTE examination at a median interval of 15 (IQR, 11.3-27.7) months and were included in the serial analysis. A total of 81.9% of patients (7208 of 8810) had stable Agile 3+ scores and 92.6% of patients (8163 of 8810) had stable Agile 4 scores (same risk categories at both assessments). The incidence of LREs was 0.6 per 1000 person-years in patients with persistently low Agile 3+ scores and 30.1 per 1000 person-years in patients with persistently high Agile 3+ scores. In patients with high Agile 3+ score at baseline, a decrease in the score by more than 20% was associated with substantial reduction in the risk of LREs. A similar trend was observed for the Agile 4 score, although it missed more LREs in the low-risk group.
Conclusions and relevance: Findings of this study suggest that single or serial Agile scores are highly accurate in predicting LREs in patients with MASLD, making them suitable alternatives to liver biopsy in routine clinical practice and in phase 2b and 3 clinical trials for steatohepatitis.
Conflict of interest statement
Figures

Comment in
-
Predicting Liver-Related Outcomes in Steatotic Liver Disease.JAMA. 2024 Apr 16;331(15):1274-1275. doi: 10.1001/jama.2024.0799. JAMA. 2024. PMID: 38512225 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

