Excess glucocorticoids inhibit murine bone turnover via modulating the immunometabolism of the skeletal microenvironment
- PMID: 38512413
- PMCID: PMC11093612
- DOI: 10.1172/JCI166795
Excess glucocorticoids inhibit murine bone turnover via modulating the immunometabolism of the skeletal microenvironment
Abstract
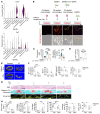
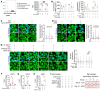
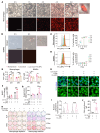
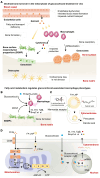
Elevated bone resorption and diminished bone formation have been recognized as the primary features of glucocorticoid-associated skeletal disorders. However, the direct effects of excess glucocorticoids on bone turnover remain unclear. Here, we explored the outcomes of exogenous glucocorticoid treatment on bone loss and delayed fracture healing in mice and found that reduced bone turnover was a dominant feature, resulting in a net loss of bone mass. The primary effect of glucocorticoids on osteogenic differentiation was not inhibitory; instead, they cooperated with macrophages to facilitate osteogenesis. Impaired local nutrient status - notably, obstructed fatty acid transportation - was a key factor contributing to glucocorticoid-induced impairment of bone turnover in vivo. Furthermore, fatty acid oxidation in macrophages fueled the ability of glucocorticoid-liganded receptors to enter the nucleus and then promoted the expression of BMP2, a key cytokine that facilitates osteogenesis. Metabolic reprogramming by localized fatty acid delivery partly rescued glucocorticoid-induced pathology by restoring a healthier immune-metabolic milieu. These data provide insights into the multifactorial metabolic mechanisms by which glucocorticoids generate skeletal disorders, thus suggesting possible therapeutic avenues.
Keywords: Bone biology; Bone disease; Fatty acid oxidation; Osteoporosis.
Conflict of interest statement
Figures








References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

