Anti-cytomegalovirus antibody levels stratify human immune profiles across the lifespan
- PMID: 38512581
- PMCID: PMC11336022
- DOI: 10.1007/s11357-024-01124-0
Anti-cytomegalovirus antibody levels stratify human immune profiles across the lifespan
Abstract
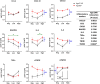
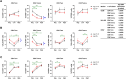
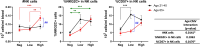
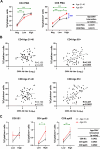
Human cytomegalovirus (hCMV) is a ubiquitous latent persistent herpesvirus infecting 60-90% of the population worldwide. hCMV carriage in immunocompetent people is asymptomatic; thus, hCMV can be considered a component of normative aging. However, hCMV powerfully modulates many features of the immune, and likely other, systems and organs. Questions remain as to how hCMV carriage affects the human host. We used anti-CMV antibody titers as a stratifying criterion to examine the impact of "intensity" of hCMV infection as a potential biomarker of aging, inflammation, and immune homeostasis in a cohort of 247 participants stratified into younger (21-40 years) and older (> 65 years of age) groups. We showed that anti-CMV antibody titers increased with age and directly correlated to increased levels of soluble tumor necrosis factor (sTNFR) I in younger but not older participants. CD8 + cell numbers were reduced in the older group due to the loss in CD8 + T naïve (Tn) cells. In CMV carriers and, in particular, in anti-CMV Ab-high participants, this loss was mitigated or reversed by an increase in the numbers of CD8 + T effector memory (Tem) and T effector memory reexpressing CD45RA (Temra) cells. Analysis of CD38, HLA-DR, and CD57 expression revealed subset (CD4 or CD8)-specific changes that correlated with anti-CMV Ab levels. In addition, anti-CMV Ab levels predicted anti-CMV CD8 T cell responsiveness to different CMV open reading frames (ORFs) selectively in older participants, which correlated to the transcriptional order of expression of specific CMV ORFs. Implications of these results for the potential predictive value of anti-CMV Ab titers during aging are discussed.
Keywords: CMV; Immune aging; Immune profiles.
© 2024. The Author(s), under exclusive licence to American Aging Association.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Chen S, Pawelec G, Trompet S, Goldeck D, Mortensen LH, Slagboom PE, Christensen K, Gussekloo J, Kearney P, Buckley BM, Ford I, Jukema JW, Westendorp RGJ, Maier AB. Associations of cytomegalovirus infection with all-cause and cardiovascular mortality in multiple observational cohort studies of older adults. J Infect Dis. 2021;223(2):238–46. 10.1093/infdis/jiaa480. 10.1093/infdis/jiaa480 - DOI - PMC - PubMed
-
- Nelson CT, Demmler GJ. Cytomegalovirus infection in the pregnant mother, fetus, and newborn infant. Clin Perinatol. 1997;24(1):151–60 https://www.ncbi.nlm.nih.gov/pubmed/9099507. 10.1016/S0095-5108(18)30189-1 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

