Dietary pyruvate targets cytosolic phospholipase A2 to mitigate inflammation and obesity in mice
- PMID: 38512816
- PMCID: PMC11365557
- DOI: 10.1093/procel/pwae014
Dietary pyruvate targets cytosolic phospholipase A2 to mitigate inflammation and obesity in mice
Abstract
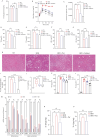
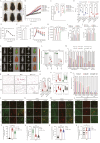
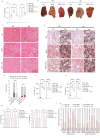
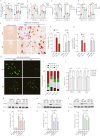
Obesity has a multifactorial etiology and is known to be a state of chronic low-grade inflammation, known as meta-inflammation. This state is associated with the development of metabolic disorders such as glucose intolerance and nonalcoholic fatty liver disease. Pyruvate is a glycolytic metabolite and a crucial node in various metabolic pathways. However, its role and molecular mechanism in obesity and associated complications are obscure. In this study, we reported that pyruvate substantially inhibited adipogenic differentiation in vitro and its administration significantly prevented HFD-induced weight gain, white adipose tissue inflammation, and metabolic dysregulation. To identify the target proteins of pyruvate, drug affinity responsive target stability was employed with proteomics, cellular thermal shift assay, and isothermal drug response to detect the interactions between pyruvate and its molecular targets. Consequently, we identified cytosolic phospholipase A2 (cPLA2) as a novel molecular target of pyruvate and demonstrated that pyruvate restrained diet-induced obesity, white adipose tissue inflammation, and hepatic steatosis in a cPLA2-dependent manner. Studies with global ablation of cPLA2 in mice showed that the protective effects of pyruvate were largely abrogated, confirming the importance of pyruvate/cPLA2 interaction in pyruvate attenuation of inflammation and obesity. Overall, our study not only establishes pyruvate as an antagonist of cPLA2 signaling and a potential therapeutic option for obesity but it also sheds light on the mechanism of its action. Pyruvate's prior clinical use indicates that it can be considered a safe and viable alternative for obesity, whether consumed as a dietary supplement or as part of a regular diet.
Keywords: cytosolic phospholipase; metabolic disease; obesity; pyruvate.
© The Author(s) 2024. Published by Oxford University Press on behalf of Higher Education Press.
Conflict of interest statement
The authors declare no competing interests related to this article.
Figures



References
-
- Cancello R, Henegar C, Viguerie Net al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005;54:2277–2286. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

