Cost-utility analysis of atezolizumab combined with bevacizumab for unresectable hepatocellular carcinoma in Thailand
- PMID: 38512900
- PMCID: PMC10956810
- DOI: 10.1371/journal.pone.0300327
Cost-utility analysis of atezolizumab combined with bevacizumab for unresectable hepatocellular carcinoma in Thailand
Abstract
Background: Clinical trials have proven the efficacy and safety of atezolizumab combined with bevacizumab (A+B) in treating unresectable hepatocellular carcinoma (uHCC). This study aimed to assess the cost-utility of A+B compared to best supportive care (BSC) among uHCC patients in Thailand.
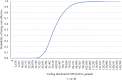
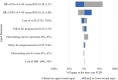
Methods: We conducted a cost-utility analysis from a societal perspective. We used a three-state Markov model to estimate relevant costs and health outcomes over the lifetime horizon. Local cost and utility data from Thai patients were applied. All costs were adjusted to 2023 values using the consumer price index. We reported results as incremental cost-effectiveness ratios (ICERs) in United States dollars ($) per quality-adjusted life year (QALY) gained. We discounted future costs and outcomes at 3% per annum. We then performed one-way sensitivity analysis and probabilistic sensitivity analysis to assess parameter uncertainty. The budget impact was conducted to estimate the financial burden from the governmental perspective over a five-year period.
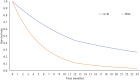
Results: Compared to BSC, A+B provided a better health benefit with 0.8309 QALY gained at an incremental lifetime cost of $45,357. The ICER was $54,589 per QALY gained. The result was sensitive to the hazard ratios for the overall survival and progression-free survival of A+B. At the current Thai willingness-to-pay (WTP) threshold of $4,678 per QALY gained, the ICER of A+B remained above the threshold. The projected budgetary requirements for implementing A+B in the respective first and fifth years would range from 8.2 to 27.9 million USD.
Conclusion: Although A+B yielded the highest clinical benefit compared with BSC for the treatment of uHCC patients, A+B is not cost-effective in Thailand at the current price and poses budgetary challenges.
Copyright: © 2024 Sriphoosanaphan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interest exist.
Figures


Similar articles
-
Cost-effectiveness of Atezolizumab Plus Bevacizumab vs Sorafenib for Patients With Unresectable or Metastatic Hepatocellular Carcinoma.JAMA Netw Open. 2021 Apr 1;4(4):e214846. doi: 10.1001/jamanetworkopen.2021.4846. JAMA Netw Open. 2021. PMID: 33825837 Free PMC article.
-
Cost-effectiveness of Atezolizumab Plus Bevacizumab vs Sorafenib as First-Line Treatment of Unresectable Hepatocellular Carcinoma.JAMA Netw Open. 2021 Feb 1;4(2):e210037. doi: 10.1001/jamanetworkopen.2021.0037. JAMA Netw Open. 2021. PMID: 33625508 Free PMC article.
-
Atezolizumab and bevacizumab combination compared with sorafenib as the first-line systemic treatment for patients with unresectable hepatocellular carcinoma: A cost-effectiveness analysis in China and the United states.Liver Int. 2021 May;41(5):1097-1104. doi: 10.1111/liv.14795. Epub 2021 Feb 8. Liver Int. 2021. PMID: 33556230 Clinical Trial.
-
Is Reconstruction of Unstable Midfoot Charcot Neuroarthropathy Cost Effective from a US Payer's Perspective?Clin Orthop Relat Res. 2020 Dec;478(12):2869-2888. doi: 10.1097/CORR.0000000000001416. Clin Orthop Relat Res. 2020. PMID: 32694315 Free PMC article.
-
Economic Evaluation of Direct Oral Anticoagulants Compared to Warfarin for Venous Thromboembolism in Thailand: A Cost-Utility Analysis.Int J Environ Res Public Health. 2023 Feb 11;20(4):3176. doi: 10.3390/ijerph20043176. Int J Environ Res Public Health. 2023. PMID: 36833871 Free PMC article. Review.
Cited by
-
Exploring the efficacy of fluorouracil and platinum based chemotherapy in advanced hepatocellular carcinoma to bridge the treatment gap in resource limited settings.Sci Rep. 2025 Jan 27;15(1):3386. doi: 10.1038/s41598-025-86523-9. Sci Rep. 2025. PMID: 39870720 Free PMC article.
-
Cost-effectiveness of immune checkpoint inhibitors as a first-line therapy for advanced hepatocellular carcinoma: a systematic review.Health Econ Rev. 2024 Jul 5;14(1):48. doi: 10.1186/s13561-024-00526-2. Health Econ Rev. 2024. PMID: 38967718 Free PMC article. Review.
References
-
- World Health Organization: International Agency for Research on Cancer (IARC). Liver: Cancer incidence and mortality statistics worldwide 2020 [updated December 2020; cited 2023 June 28]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf.
-
- Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, et al.. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13(3):414–20. Epub 2007/01/19. doi: 10.3748/wjg.v13.i3.414 ; PubMed Central PMCID: PMC4065897. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

