The role of GAPDH in the selective toxicity of CNP in melanoma cells
- PMID: 38512909
- PMCID: PMC10956844
- DOI: 10.1371/journal.pone.0300718
The role of GAPDH in the selective toxicity of CNP in melanoma cells
Abstract
Background: Malignant melanoma is the most aggressive form of skin cancer with a rather poor prognosis. Standard chemotherapy often results in severe side effects on normal (healthy) cells finally being difficult to tolerate for the patients. Shown by us earlier, cerium oxide nanoparticles (CNP, nanoceria) selectively killed A375 melanoma cells while not being cytotoxic at identical concentrations on non-cancerous cells. In conclusion, the redox-active CNP exhibited both prooxidative as well as antioxidative properties. In that context, CNP induced mitochondrial dysfunction in the studied melanoma cells via generation of reactive oxygene species (primarily hydrogen peroxide (H2O2)), but that does not account for 100% of the toxicity.
Aim: Cancer cells often show an increased glycolytic rate (Warburg effect), therefore we focused on CNP mediated changes of the glucose metabolism.
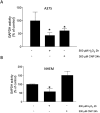
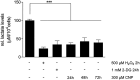
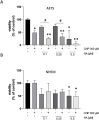
Results: It has been shown before that glyceraldehyde 3-phosphate dehydrogenase (GAPDH) activity is regulated via oxidation of a cysteine in the active center of the enzyme with a subsequent loss of activity. Upon CNP treatment, formation of cellular lactate and GAPDH activity were significantly lowered. The treatment of melanoma cells and melanocytes with the GAPDH inhibitor heptelidic acid (HA) decreased viability to a much higher extent in the cancer cells than in the studied normal (healthy) cells, highlighting and supporting the important role of GAPDH in cancer cells.
Conclusion: We identified glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a target protein for CNP mediated thiol oxidation.
Copyright: © 2024 von Montfort et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Del Fiore P, Russo I, Ferrazzi B, Monico AD, Cavallin F, Filoni A, et al. Melanoma in Adolescents and Young Adults: Evaluation of the Characteristics, Treatment Strategies, and Prognostic Factors in a Monocentric Retrospective Study. Front Oncol. 2021;11: 725523. doi: 10.3389/fonc.2021.725523 - DOI - PMC - PubMed
-
- Gagnon J, Fromm KM. Toxicity and Protective Effects of Cerium Oxide Nanoparticles (Nanoceria) Depending on Their Preparation Method, Particle Size, Cell Type, and Exposure Route. Eur J Inorg Chem. 2015;2015: 4510–4517. doi: 10.1002/ejic.201500643 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

