COVID-19 in non-hospitalised adults caused by either SARS-CoV-2 sub-variants Omicron BA.1, BA.2, BA.4/5 or Delta associates with similar illness duration, symptom severity and viral kinetics, irrespective of vaccination history
- PMID: 38512960
- PMCID: PMC10956747
- DOI: 10.1371/journal.pone.0294897
COVID-19 in non-hospitalised adults caused by either SARS-CoV-2 sub-variants Omicron BA.1, BA.2, BA.4/5 or Delta associates with similar illness duration, symptom severity and viral kinetics, irrespective of vaccination history
Abstract
Background: SARS-CoV-2 variant Omicron rapidly evolved over 2022, causing three waves of infection due to sub-variants BA.1, BA.2 and BA.4/5. We sought to characterise symptoms and viral loads over the course of COVID-19 infection with these sub-variants in otherwise-healthy, vaccinated, non-hospitalised adults, and compared data to infections with the preceding Delta variant of concern (VOC).
Methods: In a prospective, observational cohort study, healthy vaccinated UK adults who reported a positive polymerase chain reaction (PCR) or lateral flow test, self-swabbed on alternate weekdays until day 10. We compared participant-reported symptoms and viral load trajectories between infections caused by VOCs Delta and Omicron (sub-variants BA.1, BA.2 or BA.4/5), and tested for relationships between vaccine dose, symptoms and PCR cycle threshold (Ct) as a proxy for viral load using Chi-squared (χ2) and Wilcoxon tests.
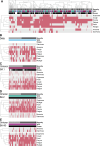
Results: 563 infection episodes were reported among 491 participants. Across infection episodes, there was little variation in symptom burden (4 [IQR 3-5] symptoms) and duration (8 [IQR 6-11] days). Whilst symptom profiles differed among infections caused by Delta compared to Omicron sub-variants, symptom profiles were similar between Omicron sub-variants. Anosmia was reported more frequently in Delta infections after 2 doses compared with Omicron sub-variant infections after 3 doses, for example: 42% (25/60) of participants with Delta infection compared to 9% (6/67) with Omicron BA.4/5 (χ2 P < 0.001; OR 7.3 [95% CI 2.7-19.4]). Fever was less common with Delta (20/60 participants; 33%) than Omicron BA.4/5 (39/67; 58%; χ2 P = 0.008; OR 0.4 [CI 0.2-0.7]). Amongst infections with an Omicron sub-variants, symptoms of coryza, fatigue, cough and myalgia predominated. Viral load trajectories and peaks did not differ between Delta, and Omicron, irrespective of symptom severity (including asymptomatic participants), VOC or vaccination status. PCR Ct values were negatively associated with time since vaccination in participants infected with BA.1 (β = -0.05 (CI -0.10-0.01); P = 0.031); however, this trend was not observed in BA.2 or BA.4/5 infections.
Conclusion: Our study emphasises both the changing symptom profile of COVID-19 infections in the Omicron era, and ongoing transmission risk of Omicron sub-variants in vaccinated adults.
Trial registration: NCT04750356.
Copyright: © 2024 Townsley et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
CSw reports interests unrelated to this Correspondence: grants from BMS, Ono-Pharmaceuticals, Boehringer-Ingelheim, Roche-Ventana, Pfizer and Archer Dx, unrelated to this Correspondence; personal fees from Genentech, Sarah Canon Research Institute, Medicxi, Bicycle Therapeutics, GRAIL, Amgen, AstraZeneca, BMS, Illumina, GlaxoSmithKline, MSD, and Roche-Ventana, unrelated to this Correspondence; and stock options from Apogen Biotech, Epic Biosciences, GRAIL, and Achilles Therapeutics, unrelated to this Correspondence. DLVB reports grants from AstraZeneca unrelated to this Correspondence. All other authors declare no competing interests. This declaration on competing interests does not alter our adherence to PLOS ONE policies on sharing data and materials
Figures



References
-
- Prevention CfDCa. Ending Isolation and Precautions for People with COVID-19: Interim Guidance: CDC; 2022. [updated 14/1/2022; cited 2022 31/5/2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html.
-
- Peacock TP, Brown JC, Zhou J, Thakur N, Sukhova K, Newman J, et al.. The altered entry pathway and antigenic distance of the SARS-CoV-2 Omicron variant map to separate domains of spike protein. bioRxiv. 2022:2021.12.31.474653. doi: 10.1101/2021.12.31.474653 - DOI
-
- Willett BJ, Grove J, MacLean O, Wilkie C, Logan N, De Lorenzo G, et al.. The hyper-transmissible SARS-CoV-2 Omicron variant exhibits significant antigenic change, vaccine escape and a switch in cell entry mechanism. medRxiv. 2022:2022.01.03. doi: 10.1101/2022.01.03.21268111 - DOI
-
- Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C, et al.. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397(10292):2331–3. Epub 2021/06/07. doi: 10.1016/S0140-6736(21)01290-3 ; PubMed Central PMCID: PMC8175044. - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

