IL7 increases targeted lipid nanoparticle-mediated mRNA expression in T cells in vitro and in vivo by enhancing T cell protein translation
- PMID: 38513098
- PMCID: PMC10990120
- DOI: 10.1073/pnas.2319856121
IL7 increases targeted lipid nanoparticle-mediated mRNA expression in T cells in vitro and in vivo by enhancing T cell protein translation
Abstract
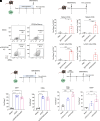
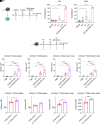
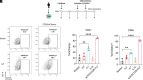
The use of lipid nanoparticles (LNP) to encapsulate and deliver mRNA has become an important therapeutic advance. In addition to vaccines, LNP-mRNA can be used in many other applications. For example, targeting the LNP with anti-CD5 antibodies (CD5/tLNP) can allow for efficient delivery of mRNA payloads to T cells to express protein. As the percentage of protein expressing T cells induced by an intravenous injection of CD5/tLNP is relatively low (4-20%), our goal was to find ways to increase mRNA-induced translation efficiency. We showed that T cell activation using an anti-CD3 antibody improved protein expression after CD5/tLNP transfection in vitro but not in vivo. T cell health and activation can be increased with cytokines, therefore, using mCherry mRNA as a reporter, we found that culturing either mouse or human T cells with the cytokine IL7 significantly improved protein expression of delivered mRNA in both CD4+ and CD8+ T cells in vitro. By pre-treating mice with systemic IL7 followed by tLNP administration, we observed significantly increased mCherry protein expression by T cells in vivo. Transcriptomic analysis of mouse T cells treated with IL7 in vitro revealed enhanced genomic pathways associated with protein translation. Improved translational ability was demonstrated by showing increased levels of protein expression after electroporation with mCherry mRNA in T cells cultured in the presence of IL7, but not with IL2 or IL15. These data show that IL7 selectively increases protein translation in T cells, and this property can be used to improve expression of tLNP-delivered mRNA in vivo.
Keywords: IL7; lipid nanoparticles; translation.
Conflict of interest statement
Competing interests statement:B.A.S. and A.B. are employees of Capstan Therapeutics. C.H.J., H.A., D.W., S.M.A., and H.P. are scientific founders and hold equity in Capstan Therapeutics. Authors have patent filings in the field of RNA therapeutics.
Figures

References
-
- Agrawal S., Garg A., Varshney V., Recent updates on applications of lipid-based nanoparticles for site-specific drug delivery. Pharm. Nanotechnol. 10, 24–41 (2022). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

