Ferredoxin reduction by hydrogen with iron functions as an evolutionary precursor of flavin-based electron bifurcation
- PMID: 38513105
- PMCID: PMC7615787
- DOI: 10.1073/pnas.2318969121
Ferredoxin reduction by hydrogen with iron functions as an evolutionary precursor of flavin-based electron bifurcation
Abstract
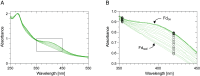
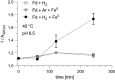
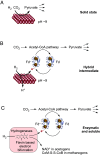
Autotrophic theories for the origin of metabolism posit that the first cells satisfied their carbon needs from CO2 and were chemolithoautotrophs that obtained their energy and electrons from H2. The acetyl-CoA pathway of CO2 fixation is central to that view because of its antiquity: Among known CO2 fixing pathways it is the only one that is i) exergonic, ii) occurs in both bacteria and archaea, and iii) can be functionally replaced in full by single transition metal catalysts in vitro. In order to operate in cells at a pH close to 7, however, the acetyl-CoA pathway requires complex multi-enzyme systems capable of flavin-based electron bifurcation that reduce low potential ferredoxin-the physiological donor of electrons in the acetyl-CoA pathway-with electrons from H2. How can the acetyl-CoA pathway be primordial if it requires flavin-based electron bifurcation? Here, we show that native iron (Fe0), but not Ni0, Co0, Mo0, NiFe, Ni2Fe, Ni3Fe, or Fe3O4, promotes the H2-dependent reduction of aqueous Clostridium pasteurianum ferredoxin at pH 8.5 or higher within a few hours at 40 °C, providing the physiological function of flavin-based electron bifurcation, but without the help of enzymes or organic redox cofactors. H2-dependent ferredoxin reduction by iron ties primordial ferredoxin reduction and early metabolic evolution to a chemical process in the Earth's crust promoted by solid-state iron, a metal that is still deposited in serpentinizing hydrothermal vents today.
Keywords: acetyl CoA pathway; origin of life; origin of metabolism; serpentinization; transition metals.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Similar articles
-
Chemical Antiquity in Metabolism.Acc Chem Res. 2024 Aug 20;57(16):2267-2278. doi: 10.1021/acs.accounts.4c00226. Epub 2024 Jul 31. Acc Chem Res. 2024. PMID: 39083571 Free PMC article.
-
Iron catalysis at the origin of life.IUBMB Life. 2017 Jun;69(6):373-381. doi: 10.1002/iub.1632. Epub 2017 May 3. IUBMB Life. 2017. PMID: 28470848
-
Native metals, electron bifurcation, and CO2 reduction in early biochemical evolution.Curr Opin Microbiol. 2018 Jun;43:77-83. doi: 10.1016/j.mib.2017.12.010. Epub 2018 Jan 12. Curr Opin Microbiol. 2018. PMID: 29316496 Review.
-
Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway.Nat Ecol Evol. 2018 Jun;2(6):1019-1024. doi: 10.1038/s41559-018-0542-2. Epub 2018 Apr 23. Nat Ecol Evol. 2018. PMID: 29686234 Free PMC article.
-
Energy conservation via electron bifurcating ferredoxin reduction and proton/Na(+) translocating ferredoxin oxidation.Biochim Biophys Acta. 2013 Feb;1827(2):94-113. doi: 10.1016/j.bbabio.2012.07.002. Epub 2012 Jul 16. Biochim Biophys Acta. 2013. PMID: 22800682 Review.
Cited by
-
Chemiosmotic ATP synthesis by minimal protocells.Cell Rep Phys Sci. 2025 Mar 19;6(3):102461. doi: 10.1016/j.xcrp.2025.102461. Cell Rep Phys Sci. 2025. PMID: 40123866 Free PMC article.
-
Bacteria on steroids.Proc Natl Acad Sci U S A. 2025 Apr;122(13):e2503396122. doi: 10.1073/pnas.2503396122. Epub 2025 Mar 24. Proc Natl Acad Sci U S A. 2025. PMID: 40127283 Free PMC article. No abstract available.
-
Microbial ecology of serpentinite-hosted ecosystems.ISME J. 2025 Jan 2;19(1):wraf029. doi: 10.1093/ismejo/wraf029. ISME J. 2025. PMID: 39961017 Free PMC article. Review.
-
Conversion of pyridoxal to pyridoxamine with NH3 and H2 on nickel generates a protometabolic nitrogen shuttle under serpentinizing conditions.FEBS J. 2025 Jun;292(12):3041-3055. doi: 10.1111/febs.17357. Epub 2024 Dec 19. FEBS J. 2025. PMID: 39703002 Free PMC article.
-
Two Key Ferredoxins for Nitrogen Fixation Have Different Specificities and Biophysical Properties.Chemistry. 2025 Jul 2;31(37):e202500844. doi: 10.1002/chem.202500844. Epub 2025 May 30. Chemistry. 2025. PMID: 40396536 Free PMC article.
References
-
- Fuchs G., Stupperich E., “Evolution of autotrophic CO2 fixation” in Evolution of Prokaryotes, Schleifer K., Stackenbrandt E., Eds. (Academic Press, London, 1985), pp. 235–251.
-
- Fuchs G., Alternative pathways of carbon dioxide fixation: Insights into the early evolution of life? Annu. Rev. Microbiol. 65, 631–658 (2011). - PubMed
-
- Wagner T., Ermler U., Shima S., The methanogenic CO2 reducing-and-fixing enzyme is bifunctional and contains 46 [4Fe-4S] clusters. Science 354, 114–117 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

