Genetic regulation of carnitine metabolism controls lipid damage repair and aging RBC hemolysis in vivo and in vitro
- PMID: 38513237
- PMCID: PMC11208298
- DOI: 10.1182/blood.2024023983
Genetic regulation of carnitine metabolism controls lipid damage repair and aging RBC hemolysis in vivo and in vitro
Abstract
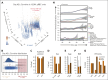
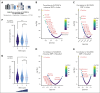
Recent large-scale multiomics studies suggest that genetic factors influence the chemical individuality of donated blood. To examine this concept, we performed metabolomics analyses of 643 blood units from volunteers who donated units of packed red blood cells (RBCs) on 2 separate occasions. These analyses identified carnitine metabolism as the most reproducible pathway across multiple donations from the same donor. We also measured l-carnitine and acyl-carnitines in 13 091 packed RBC units from donors in the Recipient Epidemiology and Donor Evaluation study. Genome-wide association studies against 879 000 polymorphisms identified critical genetic factors contributing to interdonor heterogeneity in end-of-storage carnitine levels, including common nonsynonymous polymorphisms in genes encoding carnitine transporters (SLC22A16, SLC22A5, and SLC16A9); carnitine synthesis (FLVCR1 and MTDH) and metabolism (CPT1A, CPT2, CRAT, and ACSS2), and carnitine-dependent repair of lipids oxidized by ALOX5. Significant associations between genetic polymorphisms on SLC22 transporters and carnitine pools in stored RBCs were validated in 525 Diversity Outbred mice. Donors carrying 2 alleles of the rs12210538 SLC22A16 single-nucleotide polymorphism exhibited the lowest l-carnitine levels, significant elevations of in vitro hemolysis, and the highest degree of vesiculation, accompanied by increases in lipid peroxidation markers. Separation of RBCs by age, via in vivo biotinylation in mice, and Percoll density gradients of human RBCs, showed age-dependent depletions of l-carnitine and acyl-carnitine pools, accompanied by progressive failure of the reacylation process after chemically induced membrane lipid damage. Supplementation of stored murine RBCs with l-carnitine boosted posttransfusion recovery, suggesting this could represent a viable strategy to improve RBC storage quality.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: A.D., K.C.H., and T.N. are founders of Omix Technologies Inc and Altis Biosciences LLC. A.D. and S.L.S. are scientific advisory board members for Hemanext Inc. A.D. is a scientific advisory board member for Macopharma Inc. J.C.Z. is a founder of Svalinn Therapeutics. The remaining authors declare no competing financial interests.
Figures








Comment in
-
How to digest gargantuan data on red cell aging.Blood. 2024 Jun 13;143(24):2448-2449. doi: 10.1182/blood.2024024679. Blood. 2024. PMID: 38869915 No abstract available.
References
-
- Childs B. Sir Archibald Garrod's conception of chemical individuality: a modern appreciation. N Engl J Med. 1970;282(2):71–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

