Detection of endometrial cancer in cervico-vaginal fluid and blood plasma: leveraging proteomics and machine learning for biomarker discovery
- PMID: 38513301
- PMCID: PMC10960138
- DOI: 10.1016/j.ebiom.2024.105064
Detection of endometrial cancer in cervico-vaginal fluid and blood plasma: leveraging proteomics and machine learning for biomarker discovery
Abstract
Background: The anatomical continuity between the uterine cavity and the lower genital tract allows for the exploitation of uterine-derived biomaterial in cervico-vaginal fluid for endometrial cancer detection based on non-invasive sampling methodologies. Plasma is an attractive biofluid for cancer detection due to its simplicity and ease of collection. In this biomarker discovery study, we aimed to identify proteomic signatures that accurately discriminate endometrial cancer from controls in cervico-vaginal fluid and blood plasma.
Methods: Blood plasma and Delphi Screener-collected cervico-vaginal fluid samples were acquired from symptomatic post-menopausal women with (n = 53) and without (n = 65) endometrial cancer. Digitised proteomic maps were derived for each sample using sequential window acquisition of all theoretical mass spectra (SWATH-MS). Machine learning was employed to identify the most discriminatory proteins. The best diagnostic model was determined based on accuracy and model parsimony.
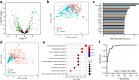
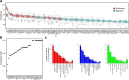
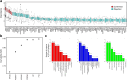
Findings: A protein signature derived from cervico-vaginal fluid more accurately discriminated cancer from control samples than one derived from plasma. A 5-biomarker panel of cervico-vaginal fluid derived proteins (HPT, LG3BP, FGA, LY6D and IGHM) predicted endometrial cancer with an AUC of 0.95 (0.91-0.98), sensitivity of 91% (83%-98%), and specificity of 86% (78%-95%). By contrast, a 3-marker panel of plasma proteins (APOD, PSMA7 and HPT) predicted endometrial cancer with an AUC of 0.87 (0.81-0.93), sensitivity of 75% (64%-86%), and specificity of 84% (75%-93%). The parsimonious model AUC values for detection of stage I endometrial cancer in cervico-vaginal fluid and blood plasma were 0.92 (0.87-0.97) and 0.88 (0.82-0.95) respectively.
Interpretation: Here, we leveraged the natural shed of endometrial tumours to potentially develop an innovative approach to endometrial cancer detection. We show proof of principle that endometrial cancers secrete unique protein signatures that can enable cancer detection via cervico-vaginal fluid assays. Confirmation in a larger independent cohort is warranted.
Funding: Cancer Research UK, Blood Cancer UK, National Institute for Health Research.
Keywords: Biomarker; Cervico-vaginal fluid; Endometrial cancer; Plasma; Proteins.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflict of interest.
Figures

References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Crosbie E.J., Kitson S.J., McAlpine J.N., Mukhopadhyay A., Powell M.E., Singh N. Endometrial cancer. Lancet. 2022;399(10333):1412–1428. - PubMed
-
- Badrick E., Cresswell K., Ellis P., et al. Top ten research priorities for detecting cancer early. Lancet Public Health. 2019;4(11) - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

