Safety and Immunogenicity of the mRNA-1273 Coronavirus Disease 2019 Vaccine in Solid Organ Transplant Recipients
- PMID: 38513368
- PMCID: PMC11420796
- DOI: 10.1093/infdis/jiae140
Safety and Immunogenicity of the mRNA-1273 Coronavirus Disease 2019 Vaccine in Solid Organ Transplant Recipients
Abstract
Background: Solid organ transplant recipients (SOTRs) are at high risk for severe COVID-19.
Methods: This open-label, phase 3b trial evaluated mRNA-1273 in 137 kidney and 77 liver SOTRs and 20 immunocompetent participants. In part A, SOTRs received three 100-µg doses of mRNA-1273; immunocompetent participants received 2 doses. In part B, an additional 100-µg dose was offered ≥4 months after the primary series. Here, we report interim trial results.
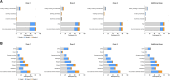
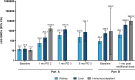
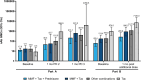
Results: mRNA-1273 was well-tolerated in SOTRs. Four serious adverse events were considered vaccine related by the investigator in 3 SOTRs with preexisting comorbidities. No vaccine-related biopsy-proven organ rejection events or deaths were reported. mRNA-1273 elicited modest neutralizing antibody responses after dose 2 and improved responses after dose 3 in SOTRs. Post-dose 3 responses among liver SOTRs were comparable to post-dose 2 responses in immunocompetent participants. Post-additional dose responses were increased in SOTRs, regardless of primary series vaccination. In liver SOTRs, post-additional dose responses were ∼3-fold higher versus post-dose 2 but lower than immunocompetent participant responses. Most kidney SOTRs received multiple immunosuppressants and had reduced antibody responses versus liver SOTRs.
Conclusions: mRNA-1273 was well-tolerated, and dose 3 and the additional dose improved antibody responses among SOTRs.
Clinical trials registration: NCT04860297.
Keywords: COVID-19; immunocompromised; mRNA-1273; solid organ transplant recipients; vaccine.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. L. F., F. P., D. S., U. S., I. L. L., E. d. W., B. G., H. Z., J. M. M., and R. D. are employees of Moderna, Inc., and may hold stock/stock options in the company. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

