Squeezing formaldehyde into C60 fullerene
- PMID: 38514674
- PMCID: PMC10957948
- DOI: 10.1038/s41467-024-46886-5
Squeezing formaldehyde into C60 fullerene
Abstract
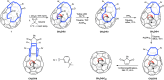
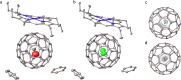
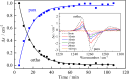
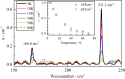
The cavity inside fullerene C60 provides a highly symmetric and inert environment for housing atoms and small molecules. Here we report the encapsulation of formaldehyde inside C60 by molecular surgery, yielding the supermolecular complex CH2O@C60, despite the 4.4 Å van der Waals length of CH2O exceeding the 3.7 Å internal diameter of C60. The presence of CH2O significantly reduces the cage HOMO-LUMO gap. Nuclear spin-spin couplings are observed between the fullerene host and the formaldehyde guest. The rapid spin-lattice relaxation of the formaldehyde 13C nuclei is attributed to a dominant spin-rotation mechanism. Despite being squeezed so tightly, the encapsulated formaldehyde molecules rotate freely about their long axes even at cryogenic temperatures, allowing observation of the ortho-to-para spin isomer conversion by infrared spectroscopy. The particle in a box nature of the system is demonstrated by the observation of two quantised translational modes in the cryogenic THz spectra.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: buckminsterfullerene. Nature. 1985;318:162–163. doi: 10.1038/318162a0. - DOI
-
- Saunders M, Cross RJ, Jiménez-Vázquez HA, Shimshi R, Khong A. Noble gas atoms inside fullerenes. Science. 1996;271:1693–1697. doi: 10.1126/science.271.5256.1693. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

