Single nuclei transcriptomics of the in situ human limbal stem cell niche
- PMID: 38514716
- PMCID: PMC10957941
- DOI: 10.1038/s41598-024-57242-4
Single nuclei transcriptomics of the in situ human limbal stem cell niche
Abstract
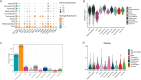
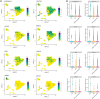
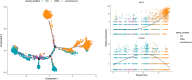
The corneal epithelium acts as a barrier to pathogens entering the eye; corneal epithelial cells are continuously renewed by uni-potent, quiescent limbal stem cells (LSCs) located at the limbus, where the cornea transitions to conjunctiva. There has yet to be a consensus on LSC markers and their transcriptome profile is not fully understood, which may be due to using cadaveric tissue without an intact stem cell niche for transcriptomics. In this study, we addressed this problem by using single nuclei RNA sequencing (snRNAseq) on healthy human limbal tissue that was immediately snap-frozen after excision from patients undergoing cataract surgery. We identified the quiescent LSCs as a sub-population of corneal epithelial cells with a low level of total transcript counts. Moreover, TP63, KRT15, CXCL14, and ITGβ4 were found to be highly expressed in LSCs and transiently amplifying cells (TACs), which constitute the corneal epithelial progenitor populations at the limbus. The surface markers SLC6A6 and ITGβ4 could be used to enrich human corneal epithelial cell progenitors, which were also found to specifically express the putative limbal progenitor cell markers MMP10 and AC093496.1.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

