Mechanism of Fat Mass and Obesity-Related Gene-Mediated Heme Oxygenase-1 m6A Modification in the Recovery of Neurological Function in Mice with Spinal Cord Injury
- PMID: 38514911
- PMCID: PMC11062882
- DOI: 10.1111/os.14002
Mechanism of Fat Mass and Obesity-Related Gene-Mediated Heme Oxygenase-1 m6A Modification in the Recovery of Neurological Function in Mice with Spinal Cord Injury
Abstract
Objectives: This study examined the mechanism of fat mass and obesity-related gene (FTO)-mediated heme oxygenase-1 (HO-1) m6A modification facilitating neurological recovery in spinal cord injury (SCI) mice. FTO/HO-1 was identified as a key regulator of SCI as well as a potential target for treatment of SCI.
Methods: An SCI mouse was treated with pcDNA3.1-FTO/pcDNA3.1-NC/Dac51. An oxygen/glucose deprivation (OGD) cell model simulated SCI, with cells treated with pcDNA3.1-FTO/si-HO-1/Dac51. Motor function and neurobehavioral evaluation were assessed using the Basso, Beattie, and Bresnahan (BBB) scale and modified neurological severity score (mNSS). Spinal cord pathology and neuronal apoptosis were assessed. Further, FTO/HO-1 mRNA and protein levels, HO-1 mRNA stability, the interaction of YTHDF2 with HO-1 mRNA, neuronal viability/apoptosis, and HO-1 m6A modification were evaluated.
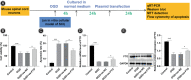
Results: Spinal cord injury mice exhibited reduced BBB, elevated mNSS scores, disorganized spinal cord cells, scattered nuclei, and severe nucleus pyknosis. pcDNA3.1-FTO elevated FTO mRNA, protein expression, and BBB score; reduced the mNSS score of SCI mice; decreased neuronal apoptosis; improved the cell arrangement; and improved nucleus pyknosis in spinal cord tissues. OGD decreased FTO expression. FTO upregulation ameliorated OGD-induced neuronal apoptosis. pcDNA3.1-FTO reduced HO-1 mRNA and protein and HO-1 m6A modification, while increasing HO-1 mRNA stability and FTO in OGD-treated cells. FTO upregulated HO-1 by modulating m6A modification. HO-1 downregulation attenuated the effect of FTO. pcDNA3.1-FTO/Dac51 increased the HO-1 m6A level in mouse spinal cord tissue homogenate, reduced BBB, boosted mNSS scores of SCI mice, aggravated nucleus pyknosis, and increased neuronal apoptosis in spinal cord tissues, confirming that FTO mediated HO-1 m6A modification facilitated neurological recovery in SCI mice.
Conclusion: The fat mass and obesity-related gene modulates HO-1 mRNA stability by regulating m6A modification levels, thereby influencing HO-1 expression and promoting neurological recovery in SCI mice.
Keywords: Fat mass and obesity‐related genes; HO‐1; Neuron; Spinal cord injury; m6A.
© 2024 The Authors. Orthopaedic Surgery published by Tianjin Hospital and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare no conflicts of interest.
Figures




Similar articles
-
Mechanism of Mettl14 regulating AIM2 inflammasome activation and neuronal apoptosis and pyroptosis in spinal cord injury by mediating PPARγ m6A methylation.J Physiol Biochem. 2024 Nov;80(4):881-894. doi: 10.1007/s13105-024-01047-6. Epub 2024 Oct 14. J Physiol Biochem. 2024. PMID: 39400644
-
FTO-mediated Nrf2 demethylation alleviates high glucose-induced oxidative stress and apoptosis in retinal pigment epithelial cells.Mol Biol Rep. 2025 Mar 7;52(1):289. doi: 10.1007/s11033-025-10400-x. Mol Biol Rep. 2025. PMID: 40053164
-
Modulation of the secondary injury process after spinal cord injury in Bach1-deficient mice by heme oxygenase-1.J Neurosurg Spine. 2008 Dec;9(6):611-20. doi: 10.3171/SPI.2008.10.08488. J Neurosurg Spine. 2008. PMID: 19035757
-
FTO m6A Demethylase in Obesity and Cancer: Implications and Underlying Molecular Mechanisms.Int J Mol Sci. 2022 Mar 30;23(7):3800. doi: 10.3390/ijms23073800. Int J Mol Sci. 2022. PMID: 35409166 Free PMC article. Review.
-
Emerging Roles of FTO in Neuropsychiatric Disorders.Biomed Res Int. 2022 Apr 26;2022:2677312. doi: 10.1155/2022/2677312. eCollection 2022. Biomed Res Int. 2022. PMID: 35528183 Free PMC article. Review.
Cited by
-
Bone marrow mesenchymal stem cells modulate miR-202-3p to suppress neuronal apoptosis following spinal cord injury through autophagy activation via the AMPK, MAPK, and PI3K/AKT/mTOR signaling pathway.Sci Rep. 2024 Dec 3;14(1):30099. doi: 10.1038/s41598-024-81332-y. Sci Rep. 2024. PMID: 39627300 Free PMC article.
References
-
- Quadri SA, Farooqui M, Ikram A, Zafar A, Khan MA, Suriya SS, et al. Recent update on basic mechanisms of spinal cord injury. Neurosurg Rev. 2020;43(2):425–441. - PubMed
-
- He N, Zheng X, He T, Shen G, Wang K, Hu J, et al. MCC950 reduces neuronal apoptosis in spinal cord injury in mice. CNS Neurol Disord Drug Targets. 2021;20(3):298–308. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

