Bioprinting Soft 3D Models of Hematopoiesis using Natural Silk Fibroin-Based Bioink Efficiently Supports Platelet Differentiation
- PMID: 38514919
- PMCID: PMC11095152
- DOI: 10.1002/advs.202308276
Bioprinting Soft 3D Models of Hematopoiesis using Natural Silk Fibroin-Based Bioink Efficiently Supports Platelet Differentiation
Abstract
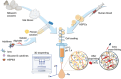
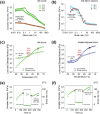
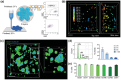
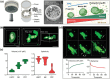
Hematopoietic stem and progenitor cells (HSPCs) continuously generate platelets throughout one's life. Inherited Platelet Disorders affect ≈ 3 million individuals worldwide and are characterized by defects in platelet formation or function. A critical challenge in the identification of these diseases lies in the absence of models that facilitate the study of hematopoiesis ex vivo. Here, a silk fibroin-based bioink is developed and designed for 3D bioprinting. This bioink replicates a soft and biomimetic environment, enabling the controlled differentiation of HSPCs into platelets. The formulation consisting of silk fibroin, gelatin, and alginate is fine-tuned to obtain a viscoelastic, shear-thinning, thixotropic bioink with the remarkable ability to rapidly recover after bioprinting and provide structural integrity and mechanical stability over long-term culture. Optical transparency allowed for high-resolution imaging of platelet generation, while the incorporation of enzymatic sensors allowed quantitative analysis of glycolytic metabolism during differentiation that is represented through measurable color changes. Bioprinting patient samples revealed a decrease in metabolic activity and platelet production in Inherited Platelet Disorders. These discoveries are instrumental in establishing reference ranges for classification and automating the assessment of treatment responses. This model has far-reaching implications for application in the research of blood-related diseases, prioritizing drug development strategies, and tailoring personalized therapies.
Keywords: bioprinting; bone marrow; eltrombopag; hematopoiesis; megakaryocyte; platelet; silk.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
C.A.D.B., V.K., P‐A.L., I.N.R., and A.B. have submitted a patent application associated with this work. The other authors declare no conflict of interest.
Figures

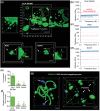
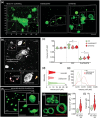

References
-
- a) Abbonante V., Di Buduo C. A., Gruppi C., De Maria C., Spedden E., De Acutis A., Staii C., Raspanti M., Vozzi G., Kaplan D. L., Moccia F., Ravid K., Balduini A., Haematologica 2017, 102, 1150; - PMC - PubMed
- b) Chen X., Hughes R., Mullin N., Hawkins R. J., Holen I., Brown N. J., Hobbs J. K., Biophys. J. 2020, 119, 502; - PMC - PubMed
- c) Vining K. H., Marneth A. E., Adu‐Berchie K., Grolman J. M., Tringides C. M., Liu Y., Wong W. J., Pozdnyakova O., Severgnini M., Stafford A., Duda G. N., Hodi F. S., Mullally A., Wucherpfennig K. W., Mooney D. J., Nat. Mater. 2022, 21, 939; - PMC - PubMed
- d) Ivanovska I. L., Shin J. W., Swift J., Discher D. E., Trends Cell Biol. 2015, 25, 523. - PMC - PubMed
-
- a) Adamo L., Naveiras O., Wenzel P. L., McKinney‐Freeman S., Mack P. J., Gracia‐Sancho J., Suchy‐Dicey A., Yoshimoto M., Lensch M. W., Yoder M. C., García‐Cardeña G., Daley G. Q., Nature 2009, 459, 1131; - PMC - PubMed
- b) Zhang P., Zhang C., Li J., Han J., Liu X., Yang H., Stem Cell Res. Ther. 2019, 10, 327; - PMC - PubMed
- c) Li H., Luo Q., Shan W., Cai S., Tie R., Xu Y., Lin Y., Qian P., Huang H., Cell. Mol. Life Sci. 2021, 78, 5881. - PMC - PubMed
-
- a) Abbonante V., Karkempetzaki A. I., Leon C., Krishnan A., Huang N., Di Buduo C. A., Cattaneo D., Ward C. M., Matsuura S., Guinard I., Weber J., De Acutis A., Vozzi G., Iurlo A., Ravid K., Balduini A., Am J. Hematol. 2024, 99, 339; - PMC - PubMed
- b) De Belly H., Paluch E. K., Chalut K. J., Nat. Rev. Mol. Cell Biol. 2022, 23, 465. - PubMed
-
- a) Di Buduo C. A., Aguilar A., Soprano P. M., Bocconi A., Miguel C. P., Mantica G., Balduini A., Haematologica 2021, 106, 947; - PMC - PubMed
- b) Currao M., Malara A., Di Buduo C. A., Abbonante V., Tozzi L., Balduini A., Exp. Cell Res. 2016, 346, 1; - PMC - PubMed
- c) Housler G. J., Miki T., Schmelzer E., Pekor C., Zhang X., Kang L., Voskinarian‐Berse V., Abbot S., Zeilinger K., Gerlach J. C., Tissue Eng Part C Methods 2012, 18, 133; - PMC - PubMed
- d) Aguilar A., Pertuy F., Eckly A., Strassel C., Collin D., Gachet C., Lanza F., Léon C., Blood 2016, 16, 2022; - PubMed
- e) Chou D. B., Frismantas V., Milton Y., David R., Pop‐Damkov P., Ferguson D., MacDonald A., Vargel Bölükbaşı Ö., Joyce C. E., Moreira Teixeira L. S., Rech A., Jiang A., Calamari E., Jalili‐Firoozinezhad S., Furlong B. A., O'Sullivan L. R., Ng C. F., Choe Y., Marquez S., Myers K. C., Weinberg O. K., Hasserjian R. P., Novak R., Levy O., Prantil‐Baun R., Novina C. D., Shimamura A., Ewart L., Ingber D. E., Nat. Biomed. Eng. 2020, 4, 394. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
