Sepsis-associated acute kidney injury: recent advances in enrichment strategies, sub-phenotyping and clinical trials
- PMID: 38515121
- PMCID: PMC10958912
- DOI: 10.1186/s13054-024-04877-4
Sepsis-associated acute kidney injury: recent advances in enrichment strategies, sub-phenotyping and clinical trials
Abstract
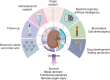
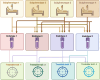
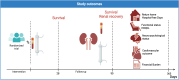
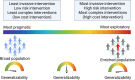
Acute kidney injury (AKI) often complicates sepsis and is associated with high morbidity and mortality. In recent years, several important clinical trials have improved our understanding of sepsis-associated AKI (SA-AKI) and impacted clinical care. Advances in sub-phenotyping of sepsis and AKI and clinical trial design offer unprecedented opportunities to fill gaps in knowledge and generate better evidence for improving the outcome of critically ill patients with SA-AKI. In this manuscript, we review the recent literature of clinical trials in sepsis with focus on studies that explore SA-AKI as a primary or secondary outcome. We discuss lessons learned and potential opportunities to improve the design of clinical trials and generate actionable evidence in future research. We specifically discuss the role of enrichment strategies to target populations that are most likely to derive benefit and the importance of patient-centered clinical trial endpoints and appropriate trial designs with the aim to provide guidance in designing future trials.
© 2024. The Author(s).
Conflict of interest statement
SMB has received fees for scientific advisory from Baxter, BioMerieux, BioPorto, Novartis, Sea Star Medical, SphingoTec. SMB is an editor of the journal. RM has received fees for scientific advisory from Baxter, AM Pharma, BioMerieux, Mallinckrodt, GE Healthcare; Sanofi; Abiomed; NovaBiomed; Novartis; Renasym; Fresenius; and Guard and had received grant support from Fresenius; Fresenius-Kabi. AZ has received fees for scientific advisory from Baxter, SphingoTec, BioMerieux, AM Pharma, Guard Therapeutics, Bayer, Novartis, Renibus, Paion and has received grant support from Fresenius, Baxter and BioMerieux. MO has been awarded research funding from Baxter, BioMerieux, Fresenius Medical and LaJolla Pharma. The funding was received by the institution. LD has received compensation from the National Kidney Foundation for her role as Deputy Editor of the American Journal of Kidney Diseases, consulting fees from AstraZeneca, Cara Therapeutics, and Merck, and compensation for serving on Data and Safety Monitoring Boards for the National Institute of Diabetes and Digestive and Kidney Diseases, and Data Monitoring Committees for CSL Behring and Vertex Pharmaceuticals. JAK is a full-time employee of Spectral Medical and has received consulting fees and grant support from BioMerieux/Astute Medical. KC is an employee of SeaStar Medical. IHS contributed to this manuscript in her personal capacity. The opinions expressed in this paper do not necessarily reflect those of the National Institute of Diabetes, Digestive and Kidney Diseases, the National Institutes of Health, the Department of Health and Human Services, and the government of the USA.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

