Heat-killed Prevotella intermedia promotes the progression of oral squamous cell carcinoma by inhibiting the expression of tumor suppressors and affecting the tumor microenvironment
- PMID: 38515216
- PMCID: PMC10956211
- DOI: 10.1186/s40164-024-00500-y
Heat-killed Prevotella intermedia promotes the progression of oral squamous cell carcinoma by inhibiting the expression of tumor suppressors and affecting the tumor microenvironment
Abstract
Background: Oral microbial dysbiosis contributes to the development of oral squamous cell carcinoma (OSCC). Our previous study showed that Prevotella intermedia (P. intermedia) were enriched in the oral mucosal surface, plaque, and saliva of patients with OSCC. Intratumoral microbiome could reshape the immune system and influence the development of various tumors. However, the invasion status of human OSCC tissues by P. intermedia and the pathway through which intratumoral P. intermedia potentiates tumor progression remain unexplored.
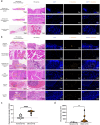
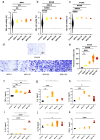
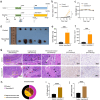
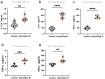
Methods: P. intermedia in human OSCC or normal tissues was detected by FISH. A mouse OSCC cell line SCC7 was adopted to investigate the effects of heat-killed P. intermedia treatment on cell proliferation, invasion, and cytokine release by using CCK-8 assay, transwell invasion assay and ELISA. Moreover, we established a mouse transplanted tumor model by using SCC7 cells, injected heat-killed P. intermedia into tumor tissues, and investigated the effects of heat-killed P. intermedia on tumor growth, invasion, cytokine levels, immune cell infiltrations, and expression levels by using gross observation, H&E staining, ELISA, immunohistochemistry, mRNA sequencing, and transcriptomic analysis.
Results: Our results indicated that P. intermedia were abundant in OSCC and surrounding muscle tissues. Heat-killed P. intermedia promoted SCC7 cell proliferation, invasion and proinflammatory cytokine secretions, accelerated transplanted tumor growth in mice, exacerbate muscle and perineural invasion of OSCC, elevated the serum levels of IL-17A, IL-6, TNF-α, IFN-γ, and PD-L1, induced Treg cells M2 type macrophages in mouse transplanted tumors. The data of transcriptomic analysis revealed that heat-killed P. intermedia increased the expression levels of inflammatory cytokines and chemokines while reduced the expression levels of some tumor suppressor genes in mouse transplanted tumors. Additionally, IL-17 signaling pathway was upregulated whereas GABAergic system was downregulated by heat-killed P. intermedia treatment.
Conclusions: Taken together, our results suggest that P. intermedia could inhibit the expression of tumor suppressors, alter the tumor microenvironment, and promote the progression of OSCC.
Keywords: Prevotella intermedia; GABAergic system; IL-17 signal pathway; Oral squamous cell carcinoma; Tumor suppressor genes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







Similar articles
-
Oral squamous cell carcinoma-derived EVs promote tumor progression by regulating inflammatory cytokines and the IL-17A-induced signaling pathway.Int Immunopharmacol. 2023 May;118:110094. doi: 10.1016/j.intimp.2023.110094. Epub 2023 Apr 6. Int Immunopharmacol. 2023. PMID: 37030119
-
Prevotella intermedia boosts OSCC progression through ISG15 upregulation: a new target for intervention.J Cancer Res Clin Oncol. 2024 Apr 21;150(4):206. doi: 10.1007/s00432-024-05730-5. J Cancer Res Clin Oncol. 2024. PMID: 38644421 Free PMC article.
-
Dysbiosis of salivary microbiome and cytokines influence oral squamous cell carcinoma through inflammation.Arch Microbiol. 2021 Jan;203(1):137-152. doi: 10.1007/s00203-020-02011-w. Epub 2020 Aug 11. Arch Microbiol. 2021. PMID: 32783067
-
Diversity and compositional differences in the oral microbiome of oral squamous cell carcinoma patients and healthy controls: a scoping review.Front Oral Health. 2024 Jun 11;5:1366153. doi: 10.3389/froh.2024.1366153. eCollection 2024. Front Oral Health. 2024. PMID: 38919733 Free PMC article.
-
The Impact of Oral Microbiome Dysbiosis on the Aetiology, Pathogenesis, and Development of Oral Cancer.Cancers (Basel). 2024 Aug 28;16(17):2997. doi: 10.3390/cancers16172997. Cancers (Basel). 2024. PMID: 39272855 Free PMC article. Review.
Cited by
-
Advancements in understanding tumor-resident bacteria and their application in cancer therapy.Mil Med Res. 2025 Jul 25;12(1):38. doi: 10.1186/s40779-025-00623-1. Mil Med Res. 2025. PMID: 40713785 Free PMC article. Review.
-
Dysbiosis and interactions of the mycobiome and bacteriome in mucosal lesions of erosive and non-erosive oral lichen planus patients.J Oral Microbiol. 2024 Jul 5;16(1):2374639. doi: 10.1080/20002297.2024.2374639. eCollection 2024. J Oral Microbiol. 2024. PMID: 38979477 Free PMC article.
-
Multivalent Immune-Protective Effects of Egg Yolk Immunoglobulin Y (IgY) Derived from Live or Inactivated Shewanella xiamenensis Against Major Aquaculture Pathogens.Int J Mol Sci. 2025 Jul 21;26(14):7012. doi: 10.3390/ijms26147012. Int J Mol Sci. 2025. PMID: 40725257 Free PMC article.
-
Lung microbiome alterations correlate with immune imbalance in non-small cell lung cancer.Front Immunol. 2025 May 14;16:1589843. doi: 10.3389/fimmu.2025.1589843. eCollection 2025. Front Immunol. 2025. PMID: 40391224 Free PMC article.
References
Grants and funding
- 0222R213, 0222C116/"3456" Cultivation Program for Junior Talents of Nanjing Stomatological School, Medical School of Nanjing University
- 81870767, 82103304/National Natural Scientific Foundation of China
- 0223A210/"2015" Cultivation Program for Reserve Talents for Academic Leaders of Nanjing Stomatological School, Medical School of Nanjing Univeristy
- QNRC2016118/Project of Jiangsu Provincial Medical Youth Talent
- 2023M741767/The Key Project of Nanjing Bureau of Science and Technology
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

