Deriving Global OH Abundance and Atmospheric Lifetimes for Long-Lived Gases: A Search for CH3CCl3 Alternatives
- PMID: 38515436
- PMCID: PMC10956888
- DOI: 10.1002/2017jd026926
Deriving Global OH Abundance and Atmospheric Lifetimes for Long-Lived Gases: A Search for CH3CCl3 Alternatives
Abstract
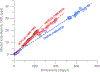
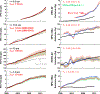
An accurate estimate of global hydroxyl radical (OH) abundance is important for projections of air quality, climate, and stratospheric ozone recovery. As the atmospheric mixing ratios of methyl chloroform (CH3CCl3) (MCF), the commonly used OH reference gas, approaches zero, it is important to find alternative approaches to infer atmospheric OH abundance and variability. The lack of global bottom-up emission inventories is the primary obstacle in choosing a MCF alternative. We illustrate that global emissions of long-lived trace gases can be inferred from their observed mixing ratio differences between the Northern Hemisphere (NH) and Southern Hemisphere (SH), given realistic estimates of their NH-SH exchange time, the emission partitioning between the two hemispheres, and the NH versus SH OH abundance ratio. Using the observed long-term trend and emissions derived from the measured hemispheric gradient, the combination of HFC-32 (CH2F2), HFC-134a (CH2FCF3, HFC-152a (CH3CHF2), and HCFC-22 (CHClF2), instead of a single gas, will be useful as a MCF alternative to infer global and hemispheric OH abundance and trace gas lifetimes. The primary assumption on which this multispecies approach relies is that the OH lifetimes can be estimated by scaling the thermal reaction rates of a reference gas at 272 K on global and hemispheric scales. Thus, the derived hemispheric and global OH estimates are forced to reconcile the observed trends and gradient for all four compounds simultaneously. However, currently, observations of these gases from the surface networks do not provide more accurate OH abundance estimate than that from MCF.
Figures





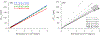

References
-
- Bednarz EM, Maycock AC, Abraham NL, Braesicke P, Dessens O, & Pyle JA (2016). Future Arctic ozone recovery: The importance of chemistry and dynamics. Atmospheric Chemistry and Physics, 16(18), 12,159–12,176. 10.5194/acp-16-12159-2016 - DOI
-
- Bousquet P, Hauglustaine DA, Peylin P, Carouge C, & Ciais P. (2005). Two decades of OH variability as inferred by an inversion of atmospheric transport and chemistry of methyl chloroform. Atmospheric Chemistry and Physics, 5(10), 2635–2656. 10.5194/acp-5-2635-2005 - DOI
-
- Bowman KP, & Erukhimova T. (2004). Comparison of global-scale Lagrangian transport properties of the NCEP reanalysis and CCM3. Journal of Climate, 17(5), 1135–1146. 10.1175/1520-0442(2004)017,1135:COGLTP.2.0.CO;2 - DOI
-
- Carpenter LJ, Reimann S, Burkholder JB, Clerbaux C, Hall BD, … Yvon-Lewis SA (2014). Ozone-depleting substances (ODSs) and other gases of interest to the Montreal Protocol, In Scientific assessment of ozone depletion: 2014, (Chapter 1, pp. 1.1–1.101). Global Ozone Research and Monitoring Project–Report No. 55, Geneva, Switzerland: World Meteorological Organization.
-
- Chipperfield MP, Liang Q, Strahan SE, Morgenstern O, Dhomse SS, Abraham NL, … Tummon F. (2014). Multi-model estimates of atmospheric lifetimes of long-lived ozone-depleting-substances: Present and future. Journal of Geophysical Research: Atmospheres, 119, 2555–2573. 10.1029/2013JD021097 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
