Inactivation of highly transmissible livestock and avian viruses including influenza A and Newcastle disease virus for molecular diagnostics
- PMID: 38515532
- PMCID: PMC10955088
- DOI: 10.3389/fvets.2024.1304022
Inactivation of highly transmissible livestock and avian viruses including influenza A and Newcastle disease virus for molecular diagnostics
Abstract
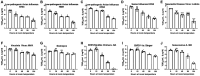
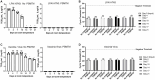
There is a critical need for an inactivation method that completely inactivates pathogens at the time of sample collection while maintaining the nucleic acid quality required for diagnostic PCR testing. This inactivation method is required to alleviate concerns about transmission potential, minimize shipping complications and cost, and enable testing in lower containment laboratories, thereby enhancing disease diagnostics through improved turn-around time. This study evaluated a panel of 10 surrogate viruses that represent highly pathogenic animal diseases. These results showed that a commercial PrimeStore® molecular transport media (PSMTM) completely inactivated all viruses tested by >99.99%, as determined by infectivity and serial passage assays. However, the detection of viral nucleic acid by qRT-PCR was comparable in PSMTM and control-treated conditions. These results were consistent when viruses were evaluated in the presence of biological material such as sera and cloacal swabs to mimic diagnostic sample conditions for non-avian and avian viruses, respectively. The results of this study may be utilized by diagnostic testing laboratories for highly pathogenic agents affecting animal and human populations. These results may be used to revise guidance for select agent diagnostic testing and the shipment of infectious substances.
Keywords: animal virus; inactivation; influenza virus; molecular diagnostics; molecular transport media.
Copyright © 2024 Welch, Shrestha, Hutchings, Pal, Levings, Robbe-Austerman, Palinski and Shanmuganatham.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Inactivation of Viable Surrogates for the Select Agents Virulent Newcastle Disease Virus and Highly Pathogenic Avian Influenza Virus Using Either Commercial Lysis Buffer or Heat.Appl Biosaf. 2019 Dec 1;24(4):189-199. doi: 10.1177/1535676019888920. Epub 2019 Dec 1. Appl Biosaf. 2019. PMID: 36032057 Free PMC article.
-
Optimal specimen collection and transport methods for the detection of avian influenza virus and Newcastle disease virus.BMC Vet Res. 2013 Feb 22;9:35. doi: 10.1186/1746-6148-9-35. BMC Vet Res. 2013. PMID: 23432911 Free PMC article.
-
Biosafety Recommendations for Work with Influenza Viruses Containing a Hemagglutinin from the A/goose/Guangdong/1/96 Lineage.MMWR Recomm Rep. 2013 Jun 28;62(RR-06):1-7. MMWR Recomm Rep. 2013. PMID: 23803973
-
Detection of animal viruses using nucleic acid sequence-based amplification (NASBA).Dev Biol (Basel). 2006;126:7-15; discussion 323. Dev Biol (Basel). 2006. PMID: 17058476 Review.
-
The evolving threat of influenza viruses of animal origin and the challenges in developing appropriate diagnostics.Clin Chem. 2012 Nov;58(11):1527-33. doi: 10.1373/clinchem.2012.182626. Epub 2012 Sep 11. Clin Chem. 2012. PMID: 22968105 Review.
Cited by
-
Effect of stabilizers on the detection of swine influenza A virus (swIAV) in spiked oral fluids over time.Porcine Health Manag. 2024 Nov 11;10(1):49. doi: 10.1186/s40813-024-00386-6. Porcine Health Manag. 2024. PMID: 39529191 Free PMC article.
-
Comparison of Extraction Methods for the Detection of Avian Influenza Virus RNA in Cattle Milk.Viruses. 2024 Sep 10;16(9):1442. doi: 10.3390/v16091442. Viruses. 2024. PMID: 39339917 Free PMC article.
-
Successful Inactivation of High-Consequence Pathogens in PrimeStore Molecular Transport Media.Viruses. 2025 Apr 29;17(5):639. doi: 10.3390/v17050639. Viruses. 2025. PMID: 40431651 Free PMC article.
References
-
- Brown VR, Miller RS, McKee SC, Ernst KH, Didero NM, Maison RM, et al. . Risks of introduction and economic consequences associated with African swine fever, classical swine fever and foot-and-mouth disease: a review of the literature. Transbound Emerg Dis. (2021) 68:1910–65. doi: 10.1111/tbed.13919, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

