Small Fluorogenic Amino Acids for Peptide-Guided Background-Free Imaging
- PMID: 38515539
- PMCID: PMC10952862
- DOI: 10.1002/ange.202216231
Small Fluorogenic Amino Acids for Peptide-Guided Background-Free Imaging
Abstract
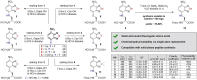
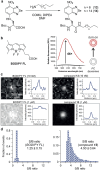
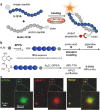
The multiple applications of super-resolution microscopy have prompted the need for minimally invasive labeling strategies for peptide-guided fluorescence imaging. Many fluorescent reporters display limitations (e.g., large and charged scaffolds, non-specific binding) as building blocks for the construction of fluorogenic peptides. Herein we have built a library of benzodiazole amino acids and systematically examined them as reporters for background-free fluorescence microscopy. We have identified amine-derivatized benzoselenadiazoles as scalable and photostable amino acids for the straightforward solid-phase synthesis of fluorescent peptides. Benzodiazole amino acids retain the binding capabilities of bioactive peptides and display excellent signal-to-background ratios. Furthermore, we have demonstrated their application in peptide-PAINT imaging of postsynaptic density protein-95 nanoclusters in the synaptosomes from mouse brain tissues.
Benzodiazole amino acids are excellent small building blocks for the construction of background‐free peptide probes for fluorescence imaging. We demonstrate their robustness and versatility for solid‐phase peptide synthesis, their minimally invasive character, and their compatibility with different optical imaging modalities, including super‐resolution peptide‐PAINT imaging.
Keywords: Fluorescence; Microscopy; Probes; Proteins; Super-Resolution.
© 2022 The Authors. Angewandte Chemie published by Wiley-VCH GmbH.
Conflict of interest statement
The University of Edinburgh has filed a patent covering some of the technology described in this manuscript. The company Tocris Bioscience obtained a license to commercialize compound 1 (SCOTfluor 510, fluoro), compound 12 (SCOTfluor 510 Dapa) and the Fmoc‐protected derivative of compound 7 (SCOTfluor 470 Dapa).
Figures

Similar articles
-
Small Fluorogenic Amino Acids for Peptide-Guided Background-Free Imaging.Angew Chem Int Ed Engl. 2023 Jan 23;62(4):e202216231. doi: 10.1002/anie.202216231. Epub 2022 Dec 14. Angew Chem Int Ed Engl. 2023. PMID: 36412996 Free PMC article.
-
Acid-Resistant BODIPY Amino Acids for Peptide-Based Fluorescence Imaging of GPR54 Receptors in Pancreatic Islets.Angew Chem Weinheim Bergstr Ger. 2023 May 8;135(20):e202302688. doi: 10.1002/ange.202302688. Epub 2023 Apr 13. Angew Chem Weinheim Bergstr Ger. 2023. PMID: 38516305 Free PMC article.
-
Alared: Solvatochromic and Fluorogenic Red Amino Acid for Ratiometric Live-Cell Imaging of Bioactive Peptides.Chemistry. 2024 Jun 20;30(35):e202401296. doi: 10.1002/chem.202401296. Epub 2024 May 28. Chemistry. 2024. PMID: 38641990
-
Fluorogenic probes for super-resolution microscopy.Org Biomol Chem. 2019 Jan 2;17(2):215-233. doi: 10.1039/c8ob02711k. Org Biomol Chem. 2019. PMID: 30539944 Review.
-
Fluorescent amino acids as versatile building blocks for chemical biology.Nat Rev Chem. 2020 Jun;4(6):275-290. doi: 10.1038/s41570-020-0186-z. Epub 2020 May 13. Nat Rev Chem. 2020. PMID: 37127957 Review.
References
-
- None
-
- Zhao C., Fernandez A., Avlonitis N., Vande Velde G., Bradley M., Read N. D., Vendrell M., ACS Comb. Sci. 2016, 18, 689–696; - PubMed
-
- Abdelfattah A. S., Kawashima T., Singh A., Novak O., Liu H., Shuai Y., Huang Y. C., Campagnola L., Seeman S. C., Yu J., Zheng J., Grimm J. B., Patel R., Friedrich J., Mensh B. D., Paninski L., Macklin J. J., Murphy G. J., Podgorski K., Lin B. J., Chen T. W., Turner G. C., Liu Z., Koyama M., Svoboda K., Ahrens M. B., Lavis L. D., Schreiter E. R., Science 2019, 365, 699–704; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
