Treatment response of advanced HNSCC towards immune checkpoint inhibition is associated with an activated effector memory T cell phenotype
- PMID: 38515578
- PMCID: PMC10955476
- DOI: 10.3389/fonc.2024.1333640
Treatment response of advanced HNSCC towards immune checkpoint inhibition is associated with an activated effector memory T cell phenotype
Abstract
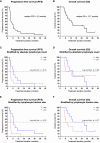
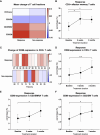
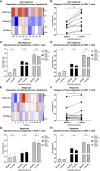
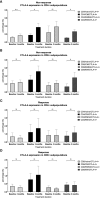
Locally advanced or metastatic head and neck squamous cell carcinoma (HNSCC) is associated with a poor prognosis. The introduction of PD-1 inhibitors has led to a significant improvement in survival, but only a subpopulation of patients responds to therapy. Current biomarkers cannot reliably identify these patients. The identification of biomarkers for the prediction and monitoring of immunotherapy is therefore of great importance. In this study, we characterized lymphocyte subsets in the peripheral blood of HNSCC patients under PD-1 inhibition. Patients with primary response (n=11) to PD-1 inhibition showed an increase of the CD3+ effector memory (CD3/EM) population and an elevated expression of the activation marker CD69 in CD3+ T cells, particularly in the CD3/EM subpopulation at 3 months when treatment response was assessed. In contrast, patients with primary treatment failure and progressive disease (n=9) despite PD-1 inhibition had lower absolute lymphocyte counts and an increased expression of CTLA-4 in CD3+ T cells at the time of treatment failure compared with baseline, particularly in CD4+ and CD8+ effector memory populations. Our results demonstrate that HNSCC patients' response to immune checkpoint inhibition shows a distinct immune signature in peripheral blood, which could help identify refractory patients earlier. Furthermore, strategies to overcome primary therapy failure by inducing a beneficial T cell phenotype or adding alternative immune checkpoint inhibitors could improve response rates and survival of HNSCC patients.
Keywords: HNSCC; PD-1; activation marker; immune checkpoint inhibition; peripheral T cells; predictive biomarker.
Copyright © 2024 Schumacher, Beer, Moraes Ribeiro, Korkmaz, Keppeler, Fitzel, Erkner, Radszuweit, Lengerke, Schneidawind, Hoefert, Mauz and Schneidawind.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

