Magnolia officinalis Rehder & E. Wilson ameliorates white adipogenesis by upregulating AMPK and SIRT1 in vitro and in vivo
- PMID: 38515723
- PMCID: PMC10955265
- DOI: 10.1016/j.heliyon.2024.e27600
Magnolia officinalis Rehder & E. Wilson ameliorates white adipogenesis by upregulating AMPK and SIRT1 in vitro and in vivo
Abstract
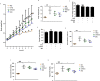
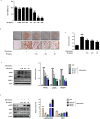
Although there is an established link between Magnolia Cortex (MO) and lipid metabolism in previous research, its exploration within the context of obesity has been limited. Therefore, the present study investigated the therapeutic effects of MO on obesity and its mechanism of action in vitro and in vivo. Our chromatography analysis revealed that Honokiol and Magnolol are contained in MO extract. In vitro experiments showed that lipid droplets, adipogenic, and lipogenic genes were notably diminished by increasing sirtuin 1 (SIRT1) and AMP-activated kinase (AMPK) protein expression in MO-treated 3T3-L1 adipocytes. In vivo experiments exhibited that MO administration significantly recovered the adipogenesis, lipogenesis, and fatty acid oxidation genes by increasing the SIRT1 and AMPK expression in white adipose tissue. Furthermore, hepatic steatosis by HFD feeding was ameliorated in MO-administered obese mice. We conclude that MO could be important manager for treating obesity through AMPK and SIRT1 regulation.
Keywords: AMP-Activated kinase; Adipose tissue; Magnolia cortex; Obesity; Sirtuin 1.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Narula S.P., Sharma P.L., Wahi P.L. Antiarrhythmic activity of racemic propranolol and its optical isomers in ouabain-induced cardiac arrhythmias in rhesus monkeys. Indian J. Med. Res. 1975;63:1705–1711. - PubMed
LinkOut - more resources
Full Text Sources

