Ferric citrate for the treatment of hyperphosphatemia and iron deficiency anaemia in patients with NDD-CKD: a systematic review and meta-analysis
- PMID: 38515853
- PMCID: PMC10955115
- DOI: 10.3389/fphar.2024.1285012
Ferric citrate for the treatment of hyperphosphatemia and iron deficiency anaemia in patients with NDD-CKD: a systematic review and meta-analysis
Abstract
Background: The application of ferric citrate therapy has yielded unexpected benefits in recent years for Chronic kidney disease patients suffering from hyperphosphatemia and iron deficiency -anaemia. Despite this, earlier research on the impact of ferric citrate on NDD-CKD has been contentious. Objective: The goal of the meta-analysis is to evaluate the evidence regarding the advantages and dangers of ferric citrate for the treatment of hyperphosphatemia and iron deficiency anaemia in NDD-CKD patients. Methods: Between the start of the study and June 2022, we searched PubMed, Embase, Cochrane, EBSCO, Scopus, Web of Science, Wan Fang Data, CNKI, and VIP databases for randomised controlled trials of iron citrate for hyperphosphatemia and anaemia in patients with NDD-CKD. For binary categorical data, risk ratios (OR) were employed, and for continuous variables, weighted mean differences The effect sizes for both count and measurement data were expressed using 95% confidence intervals Results: The meta-analysis includes eight trials with a total of 1281 NDD-CKD patients. The phosphorus-lowering effect of ferric citrate was greater compared to the control group (WMD, -0.55, 95% CI, -0.81 to -0.28; I2 = 86%, p < 0.001). Calcium (WMD, 0.092; 95% CI, -0.051 to 0.234; p > 0.05; I2 = 61.9%), PTH (WMD, -0.10; 95% CI, -0.44 to 0.23; I2 = 75%, p > 0.05) and iFGF23 (WMD, -7.62; 95% CI, -21.18 to 5.94; I2 = 20%, p > 0.05) levels were not statistically different after ferric citrate treatment compared to control treatment. Furthermore, ferric citrate increased iron reserves and haemoglobin. The ferric citrate group had considerably greater levels than the controls. Ferric citrate, on the other hand, may raise the risk of constipation, diarrhoea, and nausea. Conclusion: This meta-analysis found that ferric citrate had a beneficial effect in the treatment of NDD-CKD, particularly in reducing blood phosphorus levels when compared to a control intervention. It also shown that ferric citrate has a favourable effect on iron intake and anaemia management. In terms of safety, ferric citrate may increase the likelihood of gastrointestinal side effects.
Keywords: chronic kidney disease; ferric citrate; hyperphosphatemia; iron deficiency anaemia; meta.
Copyright © 2024 Ding, Sun, Zhang, Zhao, Lun, Liu, Zhen, Dong and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
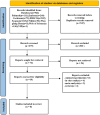
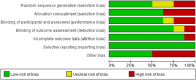




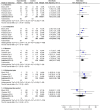
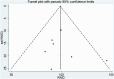
Similar articles
-
Ferric citrate in the management of hyperphosphataemia and iron deficiency anaemia: A meta-analysis in patients with chronic kidney disease.Br J Clin Pharmacol. 2021 Feb;87(2):414-426. doi: 10.1111/bcp.14396. Epub 2020 Jun 17. Br J Clin Pharmacol. 2021. PMID: 32470149
-
Ferric citrate for the treatment of hyperphosphatemia and anemia in patients with chronic kidney disease: a meta-analysis of randomized clinical trials.Ren Fail. 2022 Dec;44(1):1112-1122. doi: 10.1080/0886022X.2022.2094273. Ren Fail. 2022. PMID: 35912897 Free PMC article. Review.
-
Role of Ferric Citrate in Hyperphosphatemia and Iron Deficiency Anemia in Non Dialysis CKD Patients.J Assoc Physicians India. 2019 Apr;67(4):53-56. J Assoc Physicians India. 2019. PMID: 31311220
-
Effects of Ferric Citrate in Patients with Nondialysis-Dependent CKD and Iron Deficiency Anemia.J Am Soc Nephrol. 2017 Jun;28(6):1851-1858. doi: 10.1681/ASN.2016101053. Epub 2017 Jan 12. J Am Soc Nephrol. 2017. PMID: 28082519 Free PMC article. Clinical Trial.
-
Safe and Effective Treatment for Anemic Patients With Chronic Kidney Disease: An Updated Systematic Review and Meta-Analysis on Roxadustat.Front Pharmacol. 2021 Jul 2;12:658079. doi: 10.3389/fphar.2021.658079. eCollection 2021. Front Pharmacol. 2021. PMID: 34276361 Free PMC article.
Cited by
-
Synbiotics as a novel therapeutic approach for hyperphosphatemia and hyperparathyroidism in chronic kidney disease rats.Sci Rep. 2025 Mar 3;15(1):7493. doi: 10.1038/s41598-025-91033-9. Sci Rep. 2025. PMID: 40032932 Free PMC article.
-
Therapeutic effectiveness and safety profile of lanthanum carbonate in conjunction with calcium carbonate for managing hyperphosphatemia in hemodialysis patients: a meta-analysis of randomized controlled trials.Am J Transl Res. 2024 Nov 15;16(11):6980-6990. doi: 10.62347/WXBL2590. eCollection 2024. Am J Transl Res. 2024. PMID: 39678559 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

