Glutathione Mediates Control of Dual Differential Bio-orthogonal Labelling of Biomolecules
- PMID: 38515866
- PMCID: PMC10953330
- DOI: 10.1002/ange.202313063
Glutathione Mediates Control of Dual Differential Bio-orthogonal Labelling of Biomolecules
Abstract
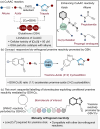
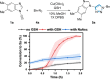
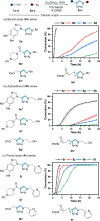
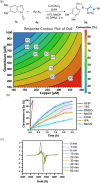
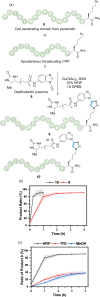
Traditional approaches to bio-orthogonal reaction discovery have focused on developing reagent pairs that react with each other faster than they are metabolically degraded. Glutathione (GSH) is typically responsible for the deactivation of most bio-orthogonal reagents. Here we demonstrate that GSH promotes a Cu-catalysed (3+2) cycloaddition reaction between an ynamine and an azide. We show that GSH acts as a redox modulator to control the Cu oxidation state in these cycloadditions. Rate enhancement of this reaction is specific for ynamine substrates and is tuneable by the Cu:GSH ratio. This unique GSH-mediated reactivity gradient is then utilised in the dual sequential bio-orthogonal labelling of peptides and oligonucleotides via two distinct chemoselective (3+2) cycloadditions.
The chemical modification at precise sites within biomolecules is essential to assist in understanding their function. The incorporation of bio‐orthogonal reactive groups provides a strategy for selective tagging, however cross‐reactivity is problematic when dual modification is required. Glutathione (GSH) is used to control the reactivity of ynamine and cyclooctyne reagents in sequential (3+2) cycloaddition reactions.
Keywords: CuAAC; bio-orthogonal chemistry; ligation; oligonucleotide; peptide.
© 2023 The Authors. Angewandte Chemie published by Wiley-VCH GmbH.
Conflict of interest statement
A patent application relating to the work in this manuscript (GB2209210.0 PG450228GB).
Figures


References
LinkOut - more resources
Full Text Sources
