Synthesis of axially chiral diaryl ethers via NHC-catalyzed atroposelective esterification
- PMID: 38516093
- PMCID: PMC10952084
- DOI: 10.1039/d3sc06444a
Synthesis of axially chiral diaryl ethers via NHC-catalyzed atroposelective esterification
Abstract
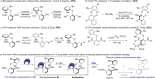
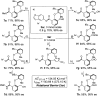
Axially chiral diaryl ethers bearing two potential axes find unique applications in bioactive molecules and catalysis. However, only very few catalytic methods have been developed to construct structurally diverse diaryl ethers. We herein describe an NHC-catalyzed atroposelective esterification of prochiral dialdehydes, leading to the construction of enantioenriched axially chiral diaryl ethers. Mechanistic studies indicate that the matched kinetic resolutions play an essential role in the challenging chiral induction of flexible dual-axial chirality by removing minor enantiomers via over-functionalization. This protocol features mild conditions, excellent enantioselectivity, broad substrate scope, and applicability to late-stage functionalization, and provides a modular platform for the synthesis of axially chiral diaryl ethers and their derivatives.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Chen Y. Yekta S. Yudin A. K. Chem. Rev. 2003;103:3155–3212. doi: 10.1021/cr020025b. - DOI - PubMed
- Akiyama T. Chem. Rev. 2007;107:5744–5758. doi: 10.1021/cr068374j. - DOI - PubMed
- Tang W. Zhang X. Chem. Rev. 2003;103:3029–3070. doi: 10.1021/cr020049i. - DOI - PubMed
- Parmar D. Sugiono E. Raja S. Rueping M. Chem. Rev. 2014;114:9047–9153. doi: 10.1021/cr5001496. - DOI - PubMed
-
- Cheng J. K. Xiang S. H. Li S. Ye L. Tan B. Chem. Rev. 2021;121:4805–4902. doi: 10.1021/acs.chemrev.0c01306. - DOI - PubMed
- Carmona J. A. Rodríguez-Franco C. Fernández R. Hornillos V. Las-saletta J. M. Chem. Soc. Rev. 2021;50:2968–2983. doi: 10.1039/D0CS00870B. - DOI - PubMed
- Cheng J. K. Xiang S. H. Tan B. Acc. Chem. Res. 2022;55:2920–2937. doi: 10.1021/acs.accounts.2c00509. - DOI - PubMed
- Zhang H. H. Shi F. Acc. Chem. Res. 2022;55:2562–2580. doi: 10.1021/acs.accounts.2c00465. - DOI - PubMed
- Qin W. Liu Y. Yan H. Acc. Chem. Res. 2022;55:2780–2795. doi: 10.1021/acs.accounts.2c00486. - DOI - PubMed
-
- Feng J. Li B. He Y. Gu Z. Angew. Chem., Int. Ed. 2016;55:2186–2190. doi: 10.1002/anie.201509571. - DOI - PubMed
- Zheng S. C. Wu S. Zhou Q. Chung L. W. Ye L. Tan B. Nat. Commun. 2017;8:15238. doi: 10.1038/ncomms15238. - DOI - PMC - PubMed
- Hu P. Hu L. Li X. X. Pan M. Lu G. Li X. Angew. Chem., Int. Ed. 2023;63:e202312923. doi: 10.1002/anie.202312923. - DOI - PubMed
LinkOut - more resources
Full Text Sources

